Email
New Issue of Px Wire: Latest product approvals, PrEP data and more
| From | AVAC <[email protected]> |
| Subject | New Issue of Px Wire: Latest product approvals, PrEP data and more |
| Date | February 14, 2023 5:03 PM |
Links have been removed from this email. Learn more in the FAQ.
Links have been removed from this email. Learn more in the FAQ.
New Issue of Px Wire: Latest product approvals, PrEP data and more
View this email in your browser ([link removed])
Px Wire ([link removed]) is AVAC’s quarterly update covering the latest in the field of biomedical HIV prevention research and development, implementation and advocacy. Each issue includes updates, emerging issues and features upcoming events.
February 14, 2023
More than 40 years into this epidemic—and in the midst of multiple pandemics and persistent inequities—2022 brought a rare opportunity to fundamentally reshape and reimagine HIV prevention, if everyone does their part.
This issue offers data from Q4 of 2022, when South Africa became the fourth country in the world to approve injectable cabotegravir (CAB) for PrEP; the dapivirine vaginal ring (DVR) ([link removed]) was approved in a growing number of countries; and oral PrEP use was on the rise ([link removed]) , passing the mark of 3.8 million initiations. For the first time, the world has multiple biomedical interventions to offer choice, and it’s essential to develop the programs that bring the fruits of science to the communities facing public health threats, while continuing to invest in developing new options to meet diverse needs.
[link removed] Check out the PDF version ([link removed]) of this issue and scroll for important updates.
------------------------------------------------------------
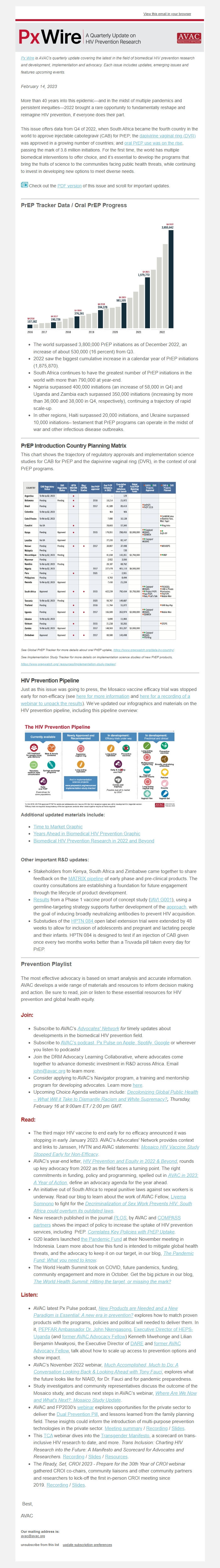
PrEP Tracker Data / Oral PrEP Progress
* The world surpassed 3,800,000 PrEP initiations as of December 2022, an increase of about 530,000 (16 percent) from Q3.
* 2022 saw the biggest cumulative increase in a calendar year of PrEP initiations (1,875,870).
* South Africa continues to have the greatest number of PrEP initiations in the world with more than 790,000 at year-end.
* Nigeria surpassed 400,000 initiations (an increase of 58,000 in Q4) and Uganda and Zambia each surpassed 350,000 initiations (increasing by more than 36,000 and 38,000 in Q4, respectively), continuing a trajectory of rapid scale-up.
* In other regions, Haiti surpassed 20,000 initiations, and Ukraine surpassed 10,000 initiations– testament that PrEP programs can operate in the midst of war and other infectious disease outbreaks.
------------------------------------------------------------
** PrEP Introduction Country Planning Matrix
------------------------------------------------------------
This chart shows the trajectory of regulatory approvals and implementation science studies for CAB for PrEP and the dapivirine vaginal ring (DVR), in the context of oral PrEP programs.
See Global PrEP Tracker for more details about oral PrEP uptake, [link removed].
See Implementation Study Tracker for more details on implementation science studies of new PrEP products, [link removed] ([link removed] ) resources/implementation-study-tracker/ ([link removed] resources/implementation-study-tracker/) .
------------------------------------------------------------
** HIV Prevention Pipeline
------------------------------------------------------------
Just as this issue was going to press, the Mosaico vaccine efficacy trial was stopped early for non-efficacy (see here for more information ([link removed]) and here for a recording of a webinar to unpack the results ([link removed]) ). We’ve updated our infographics and materials on the HIV prevention pipeline, including this pipeline overview:
Additional updated materials include:
* Time to Market Graphic ([link removed])
* Years Ahead in Biomedical HIV Prevention Graphic ([link removed])
* Biomedical HIV Prevention Research in 2022 and Beyond ([link removed])
Other important R&D updates:
* Stakeholders from Kenya, South Africa and Zimbabwe came together to share feedback on the MATRIX pipeline ([link removed]) of early phase and pre-clinical products. The country consultations are establishing a foundation for future engagement through the lifecycle of product development.
* Results ([link removed]) from a Phase 1 vaccine proof of concept study (IAVI G001 ([link removed]) ), using a germline-targeting strategy supports further development of the approach, ([link removed]) with the goal of inducing broadly neutralizing antibodies to prevent HIV acquisition.
* Substudies of the HPTN 084 ([link removed]) open label extension trial were extended by 48 weeks to allow for inclusion of adolescents and pregnant and lactating people and their infants. HPTN 084 is designed to test if an injection of CAB given once every two months works better than a Truvada pill taken every day for PrEP.
------------------------------------------------------------
Prevention Playlist
The most effective advocacy is based on smart analysis and accurate information. AVAC develops a wide range of materials and resources to inform decision making and action. Be sure to read, join or listen to these essential resources for HIV prevention and global health equity.
** Join:
------------------------------------------------------------
* Subscribe to AVAC’s Advocates' Network ([link removed]) for timely updates about developments in the biomedical HIV prevention field.
* Subscribe to AVAC’s podcast, Px Pulse on ([link removed]) Apple ([link removed]) , ([link removed]) Spotify ([link removed]) , ([link removed]) Google ([link removed]) or wherever you listen to podcasts!
* Join the DRM Advocacy Learning Collaborative, where advocates come together to advance domestic investment in R&D across Africa. Email [email protected] (mailto:[email protected]) to learn more.
* Consider applying to AVAC’s Navigator program, a training and mentorship program for developing advocates. Learn more here ([link removed]) .
* Upcoming Choice Agenda webinars include: Decolonizing Global Public Health – What Will it Take to Dismantle Racism and White Supremacy? ([link removed]) , Thursday, February 16 at 9:00am ET / 2:00 pm GMT.
** Read:
------------------------------------------------------------
* The third major HIV vaccine to end early for no efficacy announced it was stopping in early January 2023. AVAC’s Advocates' Network provides context and links to Janssen, HVTN and AVAC statements: Mosaico HIV Vaccine Study Stopped Early for Non-Efficacy ([link removed]) .
* AVAC’s year-end letter, HIV Prevention and Equity in 2022 & Beyond ([link removed]) , rounds up key advocacy from 2022 as the field faces a turning point. The right commitments in funding, policy and programming, spelled out in AVAC in 2023: A Year of Action, ([link removed]) define an advocacy agenda for the year ahead.
* An initiative out of South Africa to repeal punitive laws against sex workers is underway. Read our blog to learn about the work of AVAC Fellow, Liyema Somnono ([link removed]) to fight for the Decriminalization of Sex Work Prevents HIV: South Africa could overturn its outdated laws ([link removed]) .
* New research published in the journal PLOS ([link removed]) , by AVAC and COMPASS partners ([link removed]) shows the impact of policy to increase the uptake of HIV prevention services, including PrEP. Correlates Key Policies with PrEP Uptake ([link removed]) .
* G20 leaders launched the Pandemic Fund ([link removed]) at their November meeting in Indonesia. Learn more about how this fund is intended to mitigate global health threats, and the advocacy to keep it on our target, in our blog, The Pandemic Fund: What you need to know ([link removed]) .
* The World Health Summit took on COVID, future pandemics, funding, community engagement and more in October. Get the big picture in our blog, The World Health Summit: Hitting the target, or missing the mark? ([link removed])
** Listen:
------------------------------------------------------------
* AVAC latest Px Pulse podcast, New Products are Needed and a New Paradigm is Essential: A new era in prevention? ([link removed]) explores how to match proven products with the programs, policies and political will needed to deliver them. In it, PEPFAR Ambassador Dr. John Nkengasong ([link removed]) , Executive Director of HEPS-Uganda ([link removed]) (and former AVAC Advocacy Fellow ([link removed]) ) Kenneth Mwehonge and Lilian Benjamin Mwakyosi, the Executive Director of DARE ([link removed]) and former AVAC Advocacy Fellow ([link removed]) , talk about how to scale up access to prevention options and show impact.
* AVAC’s November 2022 webinar, Much Accomplished, Much to Do: A Conversation Looking Back & Looking Ahead with Tony Fauci ([link removed]) , explores what the future looks like for NIAID, for Dr. Fauci and for pandemic preparedness.
* Study investigators and community representatives discuss the outcome of the Mosaico study, and discuss next steps in AVAC’s webinar, Where Are We Now and What's Next?: Mosaico Study Update ([link removed]) .
* AVAC and FP2030’s webinar ([link removed]) explores opportunities for the private sector to deliver the Dual Prevention Pill ([link removed]) , and lessons learned from the family planning field. These insights could inform the introduction of multi-purpose prevention technologies in the private sector. Meeting summary ([link removed]) / Recording ([link removed]) / Slides ([link removed]) .
* This TCA ([link removed]) webinar dives into the Transgender Manifesto ([link removed]) , a scorecard on trans-inclusive HIV research to date, and more. Trans Inclusion: Charting HIV Research into the Future: A Manifesto and Scorecard for Advocates and Researchers. Recording ([link removed]) / Slides ([link removed]) / Resources ([link removed]) .
* The Ready, Set, CROI 2023 - Prepare for the 30th Year of CROI webinar gathered CROI co-chairs, community liaisons and other community partners and researchers to kick-off the first in-person CROI meeting since 2019. Recording ([link removed]) / Slides ([link removed]) .
Best,
AVAC
============================================================
Our mailing address is:
** [email protected] (mailto:[email protected])
** unsubscribe from this list ([link removed])
** update subscription preferences ([link removed])
View this email in your browser ([link removed])
Px Wire ([link removed]) is AVAC’s quarterly update covering the latest in the field of biomedical HIV prevention research and development, implementation and advocacy. Each issue includes updates, emerging issues and features upcoming events.
February 14, 2023
More than 40 years into this epidemic—and in the midst of multiple pandemics and persistent inequities—2022 brought a rare opportunity to fundamentally reshape and reimagine HIV prevention, if everyone does their part.
This issue offers data from Q4 of 2022, when South Africa became the fourth country in the world to approve injectable cabotegravir (CAB) for PrEP; the dapivirine vaginal ring (DVR) ([link removed]) was approved in a growing number of countries; and oral PrEP use was on the rise ([link removed]) , passing the mark of 3.8 million initiations. For the first time, the world has multiple biomedical interventions to offer choice, and it’s essential to develop the programs that bring the fruits of science to the communities facing public health threats, while continuing to invest in developing new options to meet diverse needs.
[link removed] Check out the PDF version ([link removed]) of this issue and scroll for important updates.
------------------------------------------------------------
PrEP Tracker Data / Oral PrEP Progress
* The world surpassed 3,800,000 PrEP initiations as of December 2022, an increase of about 530,000 (16 percent) from Q3.
* 2022 saw the biggest cumulative increase in a calendar year of PrEP initiations (1,875,870).
* South Africa continues to have the greatest number of PrEP initiations in the world with more than 790,000 at year-end.
* Nigeria surpassed 400,000 initiations (an increase of 58,000 in Q4) and Uganda and Zambia each surpassed 350,000 initiations (increasing by more than 36,000 and 38,000 in Q4, respectively), continuing a trajectory of rapid scale-up.
* In other regions, Haiti surpassed 20,000 initiations, and Ukraine surpassed 10,000 initiations– testament that PrEP programs can operate in the midst of war and other infectious disease outbreaks.
------------------------------------------------------------
** PrEP Introduction Country Planning Matrix
------------------------------------------------------------
This chart shows the trajectory of regulatory approvals and implementation science studies for CAB for PrEP and the dapivirine vaginal ring (DVR), in the context of oral PrEP programs.
See Global PrEP Tracker for more details about oral PrEP uptake, [link removed].
See Implementation Study Tracker for more details on implementation science studies of new PrEP products, [link removed] ([link removed] ) resources/implementation-study-tracker/ ([link removed] resources/implementation-study-tracker/) .
------------------------------------------------------------
** HIV Prevention Pipeline
------------------------------------------------------------
Just as this issue was going to press, the Mosaico vaccine efficacy trial was stopped early for non-efficacy (see here for more information ([link removed]) and here for a recording of a webinar to unpack the results ([link removed]) ). We’ve updated our infographics and materials on the HIV prevention pipeline, including this pipeline overview:
Additional updated materials include:
* Time to Market Graphic ([link removed])
* Years Ahead in Biomedical HIV Prevention Graphic ([link removed])
* Biomedical HIV Prevention Research in 2022 and Beyond ([link removed])
Other important R&D updates:
* Stakeholders from Kenya, South Africa and Zimbabwe came together to share feedback on the MATRIX pipeline ([link removed]) of early phase and pre-clinical products. The country consultations are establishing a foundation for future engagement through the lifecycle of product development.
* Results ([link removed]) from a Phase 1 vaccine proof of concept study (IAVI G001 ([link removed]) ), using a germline-targeting strategy supports further development of the approach, ([link removed]) with the goal of inducing broadly neutralizing antibodies to prevent HIV acquisition.
* Substudies of the HPTN 084 ([link removed]) open label extension trial were extended by 48 weeks to allow for inclusion of adolescents and pregnant and lactating people and their infants. HPTN 084 is designed to test if an injection of CAB given once every two months works better than a Truvada pill taken every day for PrEP.
------------------------------------------------------------
Prevention Playlist
The most effective advocacy is based on smart analysis and accurate information. AVAC develops a wide range of materials and resources to inform decision making and action. Be sure to read, join or listen to these essential resources for HIV prevention and global health equity.
** Join:
------------------------------------------------------------
* Subscribe to AVAC’s Advocates' Network ([link removed]) for timely updates about developments in the biomedical HIV prevention field.
* Subscribe to AVAC’s podcast, Px Pulse on ([link removed]) Apple ([link removed]) , ([link removed]) Spotify ([link removed]) , ([link removed]) Google ([link removed]) or wherever you listen to podcasts!
* Join the DRM Advocacy Learning Collaborative, where advocates come together to advance domestic investment in R&D across Africa. Email [email protected] (mailto:[email protected]) to learn more.
* Consider applying to AVAC’s Navigator program, a training and mentorship program for developing advocates. Learn more here ([link removed]) .
* Upcoming Choice Agenda webinars include: Decolonizing Global Public Health – What Will it Take to Dismantle Racism and White Supremacy? ([link removed]) , Thursday, February 16 at 9:00am ET / 2:00 pm GMT.
** Read:
------------------------------------------------------------
* The third major HIV vaccine to end early for no efficacy announced it was stopping in early January 2023. AVAC’s Advocates' Network provides context and links to Janssen, HVTN and AVAC statements: Mosaico HIV Vaccine Study Stopped Early for Non-Efficacy ([link removed]) .
* AVAC’s year-end letter, HIV Prevention and Equity in 2022 & Beyond ([link removed]) , rounds up key advocacy from 2022 as the field faces a turning point. The right commitments in funding, policy and programming, spelled out in AVAC in 2023: A Year of Action, ([link removed]) define an advocacy agenda for the year ahead.
* An initiative out of South Africa to repeal punitive laws against sex workers is underway. Read our blog to learn about the work of AVAC Fellow, Liyema Somnono ([link removed]) to fight for the Decriminalization of Sex Work Prevents HIV: South Africa could overturn its outdated laws ([link removed]) .
* New research published in the journal PLOS ([link removed]) , by AVAC and COMPASS partners ([link removed]) shows the impact of policy to increase the uptake of HIV prevention services, including PrEP. Correlates Key Policies with PrEP Uptake ([link removed]) .
* G20 leaders launched the Pandemic Fund ([link removed]) at their November meeting in Indonesia. Learn more about how this fund is intended to mitigate global health threats, and the advocacy to keep it on our target, in our blog, The Pandemic Fund: What you need to know ([link removed]) .
* The World Health Summit took on COVID, future pandemics, funding, community engagement and more in October. Get the big picture in our blog, The World Health Summit: Hitting the target, or missing the mark? ([link removed])
** Listen:
------------------------------------------------------------
* AVAC latest Px Pulse podcast, New Products are Needed and a New Paradigm is Essential: A new era in prevention? ([link removed]) explores how to match proven products with the programs, policies and political will needed to deliver them. In it, PEPFAR Ambassador Dr. John Nkengasong ([link removed]) , Executive Director of HEPS-Uganda ([link removed]) (and former AVAC Advocacy Fellow ([link removed]) ) Kenneth Mwehonge and Lilian Benjamin Mwakyosi, the Executive Director of DARE ([link removed]) and former AVAC Advocacy Fellow ([link removed]) , talk about how to scale up access to prevention options and show impact.
* AVAC’s November 2022 webinar, Much Accomplished, Much to Do: A Conversation Looking Back & Looking Ahead with Tony Fauci ([link removed]) , explores what the future looks like for NIAID, for Dr. Fauci and for pandemic preparedness.
* Study investigators and community representatives discuss the outcome of the Mosaico study, and discuss next steps in AVAC’s webinar, Where Are We Now and What's Next?: Mosaico Study Update ([link removed]) .
* AVAC and FP2030’s webinar ([link removed]) explores opportunities for the private sector to deliver the Dual Prevention Pill ([link removed]) , and lessons learned from the family planning field. These insights could inform the introduction of multi-purpose prevention technologies in the private sector. Meeting summary ([link removed]) / Recording ([link removed]) / Slides ([link removed]) .
* This TCA ([link removed]) webinar dives into the Transgender Manifesto ([link removed]) , a scorecard on trans-inclusive HIV research to date, and more. Trans Inclusion: Charting HIV Research into the Future: A Manifesto and Scorecard for Advocates and Researchers. Recording ([link removed]) / Slides ([link removed]) / Resources ([link removed]) .
* The Ready, Set, CROI 2023 - Prepare for the 30th Year of CROI webinar gathered CROI co-chairs, community liaisons and other community partners and researchers to kick-off the first in-person CROI meeting since 2019. Recording ([link removed]) / Slides ([link removed]) .
Best,
AVAC
============================================================
Our mailing address is:
** [email protected] (mailto:[email protected])
** unsubscribe from this list ([link removed])
** update subscription preferences ([link removed])
Message Analysis
- Sender: AVAC: Global Advocacy for HIV Prevention
- Political Party: n/a
- Country: n/a
- State/Locality: n/a
- Office: n/a
-
Email Providers:
- MailChimp
