| From | Megan Redshaw's Substack <[email protected]> |
| Subject | Study Shows Vitamin D Supplementation May Lower Risk of Severe COVID-19 |
| Date | January 25, 2023 12:51 PM |
Links have been removed from this email. Learn more in the FAQ.
Links have been removed from this email. Learn more in the FAQ.
View this post on the web at [link removed]
A new paper found a strong association between vitamin D supplementation and a protective effect on ICU admission in COVID-19 patients, adding more scientific support to the position that alternatives exist to vaccines that may lower one’s risk of experiencing severe disease.
In a study [ [link removed] ] published on Jan. 23 in Pharmaceuticals, researchers conducted a meta-analysis of five randomized clinical trials to determine the association between vitamin D supplementation and its effect on mortality and ICU admission in patients with COVID-19.
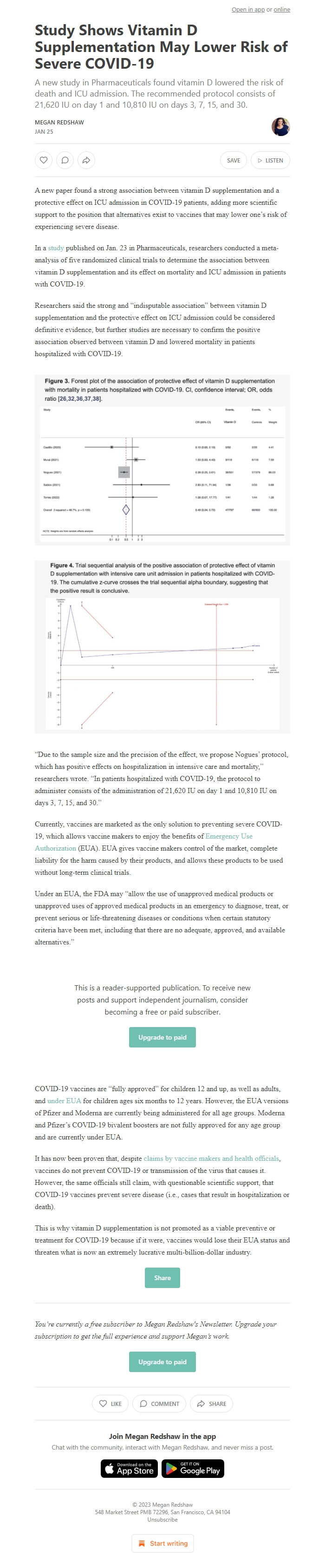
Researchers said the strong and “indisputable association” between vitamin D supplementation and the protective effect on ICU admission could be considered definitive evidence, but further studies are necessary to confirm the positive association observed between vitamin D and lowered mortality in patients hospitalized with COVID-19.
“Due to the sample size and the precision of the effect, we propose Nogues’ protocol, which has positive effects on hospitalization in intensive care and mortality,” researchers wrote. “In patients hospitalized with COVID-19, the protocol to administer consists of the administration of 21,620 IU on day 1 and 10,810 IU on days 3, 7, 15, and 30.”
Currently, vaccines are marketed as the only solution to preventing severe COVID-19, which allows vaccine makers to enjoy the benefits of Emergency Use Authorization [ [link removed] ] (EUA). EUA gives vaccine makers control of the market, complete liability for the harm caused by their products, and allows these products to be used without long-term clinical trials.
Under an EUA, the FDA may “allow the use of unapproved medical products or unapproved uses of approved medical products in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, including that there are no adequate, approved, and available alternatives.”
This is a reader-supported publication. To receive new posts and support independent journalism, consider becoming a free or paid subscriber.
COVID-19 vaccines are “fully approved” for children 12 and up, as well as adults, and under EUA [ [link removed] ] for children ages six months to 12 years. However, the EUA versions of Pfizer and Moderna are currently being administered for all age groups. Moderna and Pfizer’s COVID-19 bivalent boosters are not fully approved for any age group and are currently under EUA.
It has now been proven that, despite claims by vaccine makers and health officials [ [link removed] ], vaccines do not prevent COVID-19 or transmission of the virus that causes it. However, the same officials still claim, with questionable scientific support, that COVID-19 vaccines prevent severe disease (i.e., cases that result in hospitalization or death).
This is why vitamin D supplementation is not promoted as a viable preventive or treatment for COVID-19 because if it were, vaccines would lose their EUA status and threaten what is now an extremely lucrative multi-billion-dollar industry.
Unsubscribe [link removed]?
A new paper found a strong association between vitamin D supplementation and a protective effect on ICU admission in COVID-19 patients, adding more scientific support to the position that alternatives exist to vaccines that may lower one’s risk of experiencing severe disease.
In a study [ [link removed] ] published on Jan. 23 in Pharmaceuticals, researchers conducted a meta-analysis of five randomized clinical trials to determine the association between vitamin D supplementation and its effect on mortality and ICU admission in patients with COVID-19.
Researchers said the strong and “indisputable association” between vitamin D supplementation and the protective effect on ICU admission could be considered definitive evidence, but further studies are necessary to confirm the positive association observed between vitamin D and lowered mortality in patients hospitalized with COVID-19.
“Due to the sample size and the precision of the effect, we propose Nogues’ protocol, which has positive effects on hospitalization in intensive care and mortality,” researchers wrote. “In patients hospitalized with COVID-19, the protocol to administer consists of the administration of 21,620 IU on day 1 and 10,810 IU on days 3, 7, 15, and 30.”
Currently, vaccines are marketed as the only solution to preventing severe COVID-19, which allows vaccine makers to enjoy the benefits of Emergency Use Authorization [ [link removed] ] (EUA). EUA gives vaccine makers control of the market, complete liability for the harm caused by their products, and allows these products to be used without long-term clinical trials.
Under an EUA, the FDA may “allow the use of unapproved medical products or unapproved uses of approved medical products in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, including that there are no adequate, approved, and available alternatives.”
This is a reader-supported publication. To receive new posts and support independent journalism, consider becoming a free or paid subscriber.
COVID-19 vaccines are “fully approved” for children 12 and up, as well as adults, and under EUA [ [link removed] ] for children ages six months to 12 years. However, the EUA versions of Pfizer and Moderna are currently being administered for all age groups. Moderna and Pfizer’s COVID-19 bivalent boosters are not fully approved for any age group and are currently under EUA.
It has now been proven that, despite claims by vaccine makers and health officials [ [link removed] ], vaccines do not prevent COVID-19 or transmission of the virus that causes it. However, the same officials still claim, with questionable scientific support, that COVID-19 vaccines prevent severe disease (i.e., cases that result in hospitalization or death).
This is why vitamin D supplementation is not promoted as a viable preventive or treatment for COVID-19 because if it were, vaccines would lose their EUA status and threaten what is now an extremely lucrative multi-billion-dollar industry.
Unsubscribe [link removed]?
Message Analysis
- Sender: Megan Redshaw
- Political Party: n/a
- Country: United States
- State/Locality: n/a
- Office: n/a
