Email
Track the Science; Advance the Priorities: The 2025 Update of the People’s Research Agenda
| From | AVAC <[email protected]> |
| Subject | Track the Science; Advance the Priorities: The 2025 Update of the People’s Research Agenda |
| Date | December 11, 2025 6:00 AM |
Links have been removed from this email. Learn more in the FAQ.
Links have been removed from this email. Learn more in the FAQ.
View this email in your browser ([link removed])
[link removed] December 11, 2025
** Track the Science; Advance the Priorities: The 2025 Update of the People’s Research Agenda
------------------------------------------------------------
Dear Advocate,
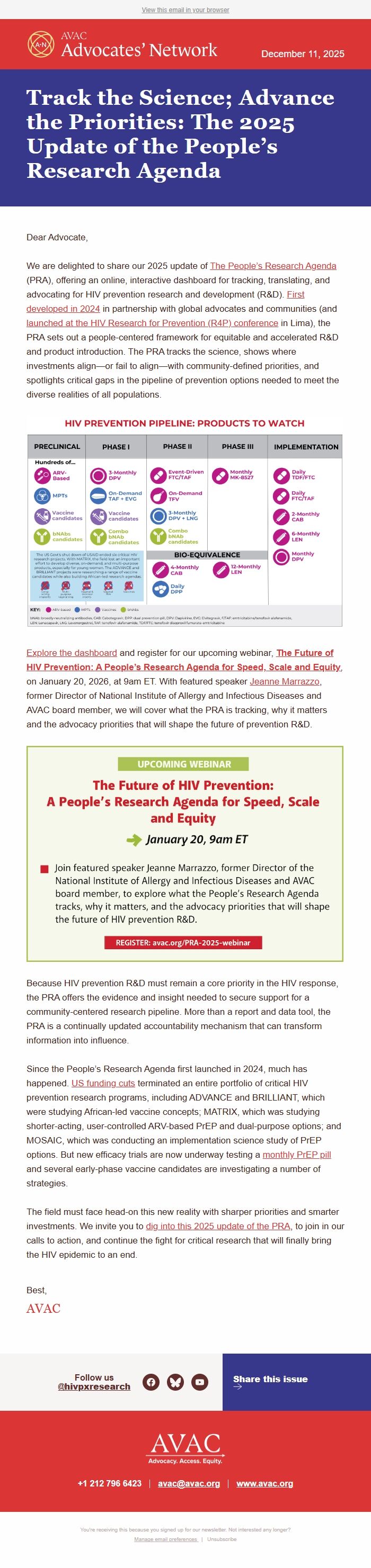
We are delighted to share our 2025 update of The People’s Research Agenda ([link removed]) (PRA), offering an online, interactive dashboard for tracking, translating, and advocating for HIV prevention research and development (R&D). First developed in 2024 ([link removed])) in partnership with global advocates and communities (and launched at the HIV Research for Prevention (R4P) conference ([link removed]) in Lima), the PRA sets out a people-centered framework for equitable and accelerated R&D and product introduction. The PRA tracks the science, shows where investments align—or fail to align—with community-defined priorities, and spotlights critical gaps in the pipeline of prevention options needed to meet the diverse realities of all populations.
[link removed]
Explore the dashboard ([link removed]) and register for our upcoming webinar, The Future of HIV Prevention: A People’s Research Agenda for Speed, Scale and Equity ([link removed]) , on January 20, 2026, at 9am ET. With featured speaker Jeanne Marrazzo ([link removed]) , former Director of National Institute of Allergy and Infectious Diseases and AVAC board member, we will cover what the PRA is tracking, why it matters and the advocacy priorities that will shape the future of prevention R&D.
[link removed]
Because HIV prevention R&D must remain a core priority in the HIV response, the PRA offers the evidence and insight needed to secure support for a community-centered research pipeline. More than a report and data tool, the PRA is a continually updated accountability mechanism that can transform information into influence.
Since the People’s Research Agenda first launched in 2024, much has happened. US funding cuts ([link removed]) terminated an entire portfolio of critical HIV prevention research programs, including ADVANCE and BRILLIANT, which were studying African-led vaccine concepts; MATRIX, which was studying shorter-acting, user-controlled ARV-based PrEP and dual-purpose options; and MOSAIC, which was conducting an implementation science study of PrEP options. But new efficacy trials are now underway testing a monthly PrEP pill ([link removed]) and several early-phase vaccine candidates are investigating a number of strategies.
The field must face head-on this new reality with sharper priorities and smarter investments. We invite you to dig into this 2025 update of the PRA ([link removed]) , to join in our calls to action, and continue the fight for critical research that will finally bring the HIV epidemic to an end.
Best,
AVAC
Follow us @hivpxresearch ([link removed])
[link removed] [link removed] [link removed]
Share this issue ([link removed])
AVAC Global Advocacy for HIV Prevention
+1 212 796 6423 [email protected] (mailto:[email protected]) www.avac.org ([link removed])
You're receiving this because you signed up for our newsletter. Not interested any longer?
Manage email preferences ([link removed]) | Unsubscribe ([link removed])
[link removed] December 11, 2025
** Track the Science; Advance the Priorities: The 2025 Update of the People’s Research Agenda
------------------------------------------------------------
Dear Advocate,
We are delighted to share our 2025 update of The People’s Research Agenda ([link removed]) (PRA), offering an online, interactive dashboard for tracking, translating, and advocating for HIV prevention research and development (R&D). First developed in 2024 ([link removed])) in partnership with global advocates and communities (and launched at the HIV Research for Prevention (R4P) conference ([link removed]) in Lima), the PRA sets out a people-centered framework for equitable and accelerated R&D and product introduction. The PRA tracks the science, shows where investments align—or fail to align—with community-defined priorities, and spotlights critical gaps in the pipeline of prevention options needed to meet the diverse realities of all populations.
[link removed]
Explore the dashboard ([link removed]) and register for our upcoming webinar, The Future of HIV Prevention: A People’s Research Agenda for Speed, Scale and Equity ([link removed]) , on January 20, 2026, at 9am ET. With featured speaker Jeanne Marrazzo ([link removed]) , former Director of National Institute of Allergy and Infectious Diseases and AVAC board member, we will cover what the PRA is tracking, why it matters and the advocacy priorities that will shape the future of prevention R&D.
[link removed]
Because HIV prevention R&D must remain a core priority in the HIV response, the PRA offers the evidence and insight needed to secure support for a community-centered research pipeline. More than a report and data tool, the PRA is a continually updated accountability mechanism that can transform information into influence.
Since the People’s Research Agenda first launched in 2024, much has happened. US funding cuts ([link removed]) terminated an entire portfolio of critical HIV prevention research programs, including ADVANCE and BRILLIANT, which were studying African-led vaccine concepts; MATRIX, which was studying shorter-acting, user-controlled ARV-based PrEP and dual-purpose options; and MOSAIC, which was conducting an implementation science study of PrEP options. But new efficacy trials are now underway testing a monthly PrEP pill ([link removed]) and several early-phase vaccine candidates are investigating a number of strategies.
The field must face head-on this new reality with sharper priorities and smarter investments. We invite you to dig into this 2025 update of the PRA ([link removed]) , to join in our calls to action, and continue the fight for critical research that will finally bring the HIV epidemic to an end.
Best,
AVAC
Follow us @hivpxresearch ([link removed])
[link removed] [link removed] [link removed]
Share this issue ([link removed])
AVAC Global Advocacy for HIV Prevention
+1 212 796 6423 [email protected] (mailto:[email protected]) www.avac.org ([link removed])
You're receiving this because you signed up for our newsletter. Not interested any longer?
Manage email preferences ([link removed]) | Unsubscribe ([link removed])
Message Analysis
- Sender: AVAC: Global Advocacy for HIV Prevention
- Political Party: n/a
- Country: n/a
- State/Locality: n/a
- Office: n/a
-
Email Providers:
- MailChimp
