Email
Sunday Science: ‘Pac-Man With a Ponytail’ Proteins Regulate Everything From Night Vision to Heartbeats – Studying What GRKs Look Like Could Improve an Array of Drugs
| From | xxxxxx <[email protected]> |
| Subject | Sunday Science: ‘Pac-Man With a Ponytail’ Proteins Regulate Everything From Night Vision to Heartbeats – Studying What GRKs Look Like Could Improve an Array of Drugs |
| Date | March 10, 2025 8:30 AM |
Links have been removed from this email. Learn more in the FAQ.
Links have been removed from this email. Learn more in the FAQ.
[[link removed]]
SUNDAY SCIENCE: ‘PAC-MAN WITH A PONYTAIL’ PROTEINS REGULATE
EVERYTHING FROM NIGHT VISION TO HEARTBEATS – STUDYING WHAT GRKS LOOK
LIKE COULD IMPROVE AN ARRAY OF DRUGS
[[link removed]]
Priyanka Naik
March 7, 2025
The Conversation
[[link removed]]
*
[[link removed]]
*
[[link removed]]
*
*
[[link removed]]
_ Each cell in your body relies on precise communication with other
cells to function properly. At the center of this process are the
molecular switches that turn communication signals in the body on and
off. _
Rhodopsin kinase – GRK1 – is a GRK found in the retina of your
eyes., Priyanka Naik
Each cell in your body relies on precise communication with other
cells to function properly. At the center of this process are the
molecular switches that turn communication signals in the body on and
off. These molecules are key players in health and disease. One such
molecular switch is G protein-coupled receptor kinases
[[link removed]], or GRKs for short.
From vision to heart function and cell growth, GRKs play a vital role
in maintaining physiological balance. When they go awry, they can
contribute to cardiovascular disease
[[link removed]], inflammatory illnesses
[[link removed]] like rheumatoid arthritis
and multiple sclerosis, neurodegenerative diseases
[[link removed]] like Alzheimer’s, and multiple
types of cancer [[link removed]].
Their involvement in a broad range of diseases makes GRKs an
attractive drug target. Around 30% to 40% of all drugs
[[link removed]] currently on the market focus on
these proteins. However, designing drugs that selectively target
specific GRKs is a difficult task. Because they are structurally
similar [[link removed]] to each other and
to other proteins, molecules binding to one GRK might also bind to
many other enzymes and cause unwanted side effects.
A better understanding of how GRKs interact with their targets can
help researchers develop better drugs. So my work
[[link removed]] in the Tesmer Lab
[[link removed]] at Purdue
University focuses on uncovering more information on the structure of
GRKs.
What do G protein-coupled receptor kinases look like?
What researchers know about the structure of GRKs has advanced
significantly over the past two decades, revealing the intricate
mechanisms by which they function.
The ability to physically look at proteins is highly useful for drug
development. Seeing a protein’s structure is like looking at a
jigsaw puzzle – you can find the missing piece by knowing its shape.
Similarly, knowing a protein’s shape helps scientists design
molecules that fit perfectly into it, making drugs more effective.
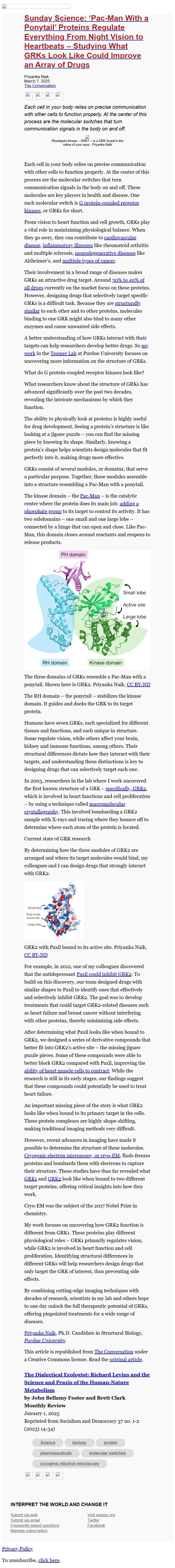
GRKs consist of several modules, or domains, that serve a particular
purpose. Together, these modules assemble into a structure resembling
a Pac-Man with a ponytail.
The kinase domain – the Pac-Man
[[link removed]] – is the
catalytic center where the protein does its main job: adding a
phosphate group
[[link removed]]
to its target to control its activity. It has two subdomains – one
small and one large lobe – connected by a hinge that can open and
close. Like Pac-Man, this domain closes around reactants and reopens
to release products.
[Array of spiral ribbons arranged into roughly three sections]
[[link removed]]
The three domains of GRKs resemble a Pac-Man with a ponytail. Shown
here is GRK2. Priyanka Naik, CC BY-ND
[[link removed]]
The RH domain – the ponytail – stabilizes the kinase domain. It
guides and docks the GRK to its target protein.
Humans have seven GRKs, each specialized for different tissues and
functions, and each unique in structure. Some regulate vision, while
others affect your brain, kidney and immune functions, among others.
Their structural differences dictate how they interact with their
targets, and understanding these distinctions is key to designing
drugs that can selectively target each one.
In 2003, researchers in the lab where I work uncovered the first known
structure of a GRK – specifically, GRK2
[[link removed]], which is involved in heart
functions and cell proliferation – by using a technique called
macromolecular crystallography
[[link removed](Analytical_Chemistry)/Instrumentation_and_Analysis/Diffraction_Scattering_Techniques/X-ray_Crystallography].
This involved bombarding a GRK2 sample with X-rays and tracing where
they bounce off to determine where each atom of the protein is
located.
Current state of GRK research
By determining how the three modules of GRK2 are arranged and where
its target molecules would bind, my colleagues and I can design drugs
that strongly interact with GRK2.
[Irregularly shaped lump resembling a drumette]
[[link removed]]
GRK2 with Paxil bound to its active site. Priyanka Naik, CC BY-ND
[[link removed]]
For example, in 2012, one of my colleagues discovered that the
antidepressant Paxil could inhibit GRK2
[[link removed]]. To build on this discovery, our
team designed drugs with similar shapes to Paxil to identify ones that
effectively and selectively inhibit GRK2. The goal was to develop
treatments that could target GRK2-related diseases such as heart
failure and breast cancer without interfering with other proteins,
thereby minimizing side effects.
After determining what Paxil looks like when bound to GRK2, we
designed a series of derivative compounds that better fit into
GRK2’s active site – the missing jigsaw puzzle pieces. Some of
these compounds were able to better block GRK2 compared with Paxil,
improving the ability of heart muscle cells to contract
[[link removed]]. While the research is
still in its early stages, our findings suggest that these compounds
could potentially be used to treat heart failure.
An important missing piece of the story is what GRK2 looks like when
bound to its primary target in the cells. These protein complexes are
highly shape-shifting, making traditional imaging methods very
difficult.
However, recent advances in imaging have made it possible to determine
the structure of these molecules. Cryogenic electron microscopy, or
cryo-EM
[[link removed]],
flash-freezes proteins and bombards them with electrons to capture
their structure. These studies have thus far revealed what GRK1
[[link removed]] and GRK2
[[link removed]] look like when bound to
two different target proteins, offering critical insights into how
they work.
Cryo-EM was the subject of the 2017 Nobel Prize in chemistry.
My work focuses on uncovering how GRK2 function is different from
GRK1. These proteins play different physiological roles – GRK1
primarily regulates vision, while GRK2 is involved in heart function
and cell proliferation. Identifying structural differences in
different GRKs will help researchers design drugs that only target the
GRK of interest, thus preventing side effects.
By combining cutting-edge imaging techniques with decades of research,
scientists in my lab and others hope to one day unlock the full
therapeutic potential of GRKs, offering pinpointed treatments for a
wide range of diseases.[The Conversation]
Priyanka Naik
[[link removed]], Ph.D.
Candidate in Structural Biology, _Purdue University
[[link removed]]_
This article is republished from The Conversation
[[link removed]] under a Creative Commons license. Read
the original article
[[link removed]].
The Dialectical Ecologist: Richard Levins and the Science and Praxis
of the Human-Nature Metabolism
[[link removed]]
by John Bellamy Foster and Brett Clark
Monthly Review
January 1, 2025
Reprinted from Socialism and Democracy 37 no. 1-2 (2023) 14-34)
* Science
[[link removed]]
* biology
[[link removed]]
* protein
[[link removed]]
* pharmaceuticals
[[link removed]]
* molecular switches
[[link removed]]
* cryogenic electron microscopy
[[link removed]]
*
[[link removed]]
*
[[link removed]]
*
*
[[link removed]]
INTERPRET THE WORLD AND CHANGE IT
Submit via web
[[link removed]]
Submit via email
Frequently asked questions
[[link removed]]
Manage subscription
[[link removed]]
Visit xxxxxx.org
[[link removed]]
Twitter [[link removed]]
Facebook [[link removed]]
[link removed]
To unsubscribe, click the following link:
[link removed]
SUNDAY SCIENCE: ‘PAC-MAN WITH A PONYTAIL’ PROTEINS REGULATE
EVERYTHING FROM NIGHT VISION TO HEARTBEATS – STUDYING WHAT GRKS LOOK
LIKE COULD IMPROVE AN ARRAY OF DRUGS
[[link removed]]
Priyanka Naik
March 7, 2025
The Conversation
[[link removed]]
*
[[link removed]]
*
[[link removed]]
*
*
[[link removed]]
_ Each cell in your body relies on precise communication with other
cells to function properly. At the center of this process are the
molecular switches that turn communication signals in the body on and
off. _
Rhodopsin kinase – GRK1 – is a GRK found in the retina of your
eyes., Priyanka Naik
Each cell in your body relies on precise communication with other
cells to function properly. At the center of this process are the
molecular switches that turn communication signals in the body on and
off. These molecules are key players in health and disease. One such
molecular switch is G protein-coupled receptor kinases
[[link removed]], or GRKs for short.
From vision to heart function and cell growth, GRKs play a vital role
in maintaining physiological balance. When they go awry, they can
contribute to cardiovascular disease
[[link removed]], inflammatory illnesses
[[link removed]] like rheumatoid arthritis
and multiple sclerosis, neurodegenerative diseases
[[link removed]] like Alzheimer’s, and multiple
types of cancer [[link removed]].
Their involvement in a broad range of diseases makes GRKs an
attractive drug target. Around 30% to 40% of all drugs
[[link removed]] currently on the market focus on
these proteins. However, designing drugs that selectively target
specific GRKs is a difficult task. Because they are structurally
similar [[link removed]] to each other and
to other proteins, molecules binding to one GRK might also bind to
many other enzymes and cause unwanted side effects.
A better understanding of how GRKs interact with their targets can
help researchers develop better drugs. So my work
[[link removed]] in the Tesmer Lab
[[link removed]] at Purdue
University focuses on uncovering more information on the structure of
GRKs.
What do G protein-coupled receptor kinases look like?
What researchers know about the structure of GRKs has advanced
significantly over the past two decades, revealing the intricate
mechanisms by which they function.
The ability to physically look at proteins is highly useful for drug
development. Seeing a protein’s structure is like looking at a
jigsaw puzzle – you can find the missing piece by knowing its shape.
Similarly, knowing a protein’s shape helps scientists design
molecules that fit perfectly into it, making drugs more effective.
GRKs consist of several modules, or domains, that serve a particular
purpose. Together, these modules assemble into a structure resembling
a Pac-Man with a ponytail.
The kinase domain – the Pac-Man
[[link removed]] – is the
catalytic center where the protein does its main job: adding a
phosphate group
[[link removed]]
to its target to control its activity. It has two subdomains – one
small and one large lobe – connected by a hinge that can open and
close. Like Pac-Man, this domain closes around reactants and reopens
to release products.
[Array of spiral ribbons arranged into roughly three sections]
[[link removed]]
The three domains of GRKs resemble a Pac-Man with a ponytail. Shown
here is GRK2. Priyanka Naik, CC BY-ND
[[link removed]]
The RH domain – the ponytail – stabilizes the kinase domain. It
guides and docks the GRK to its target protein.
Humans have seven GRKs, each specialized for different tissues and
functions, and each unique in structure. Some regulate vision, while
others affect your brain, kidney and immune functions, among others.
Their structural differences dictate how they interact with their
targets, and understanding these distinctions is key to designing
drugs that can selectively target each one.
In 2003, researchers in the lab where I work uncovered the first known
structure of a GRK – specifically, GRK2
[[link removed]], which is involved in heart
functions and cell proliferation – by using a technique called
macromolecular crystallography
[[link removed](Analytical_Chemistry)/Instrumentation_and_Analysis/Diffraction_Scattering_Techniques/X-ray_Crystallography].
This involved bombarding a GRK2 sample with X-rays and tracing where
they bounce off to determine where each atom of the protein is
located.
Current state of GRK research
By determining how the three modules of GRK2 are arranged and where
its target molecules would bind, my colleagues and I can design drugs
that strongly interact with GRK2.
[Irregularly shaped lump resembling a drumette]
[[link removed]]
GRK2 with Paxil bound to its active site. Priyanka Naik, CC BY-ND
[[link removed]]
For example, in 2012, one of my colleagues discovered that the
antidepressant Paxil could inhibit GRK2
[[link removed]]. To build on this discovery, our
team designed drugs with similar shapes to Paxil to identify ones that
effectively and selectively inhibit GRK2. The goal was to develop
treatments that could target GRK2-related diseases such as heart
failure and breast cancer without interfering with other proteins,
thereby minimizing side effects.
After determining what Paxil looks like when bound to GRK2, we
designed a series of derivative compounds that better fit into
GRK2’s active site – the missing jigsaw puzzle pieces. Some of
these compounds were able to better block GRK2 compared with Paxil,
improving the ability of heart muscle cells to contract
[[link removed]]. While the research is
still in its early stages, our findings suggest that these compounds
could potentially be used to treat heart failure.
An important missing piece of the story is what GRK2 looks like when
bound to its primary target in the cells. These protein complexes are
highly shape-shifting, making traditional imaging methods very
difficult.
However, recent advances in imaging have made it possible to determine
the structure of these molecules. Cryogenic electron microscopy, or
cryo-EM
[[link removed]],
flash-freezes proteins and bombards them with electrons to capture
their structure. These studies have thus far revealed what GRK1
[[link removed]] and GRK2
[[link removed]] look like when bound to
two different target proteins, offering critical insights into how
they work.
Cryo-EM was the subject of the 2017 Nobel Prize in chemistry.
My work focuses on uncovering how GRK2 function is different from
GRK1. These proteins play different physiological roles – GRK1
primarily regulates vision, while GRK2 is involved in heart function
and cell proliferation. Identifying structural differences in
different GRKs will help researchers design drugs that only target the
GRK of interest, thus preventing side effects.
By combining cutting-edge imaging techniques with decades of research,
scientists in my lab and others hope to one day unlock the full
therapeutic potential of GRKs, offering pinpointed treatments for a
wide range of diseases.[The Conversation]
Priyanka Naik
[[link removed]], Ph.D.
Candidate in Structural Biology, _Purdue University
[[link removed]]_
This article is republished from The Conversation
[[link removed]] under a Creative Commons license. Read
the original article
[[link removed]].
The Dialectical Ecologist: Richard Levins and the Science and Praxis
of the Human-Nature Metabolism
[[link removed]]
by John Bellamy Foster and Brett Clark
Monthly Review
January 1, 2025
Reprinted from Socialism and Democracy 37 no. 1-2 (2023) 14-34)
* Science
[[link removed]]
* biology
[[link removed]]
* protein
[[link removed]]
* pharmaceuticals
[[link removed]]
* molecular switches
[[link removed]]
* cryogenic electron microscopy
[[link removed]]
*
[[link removed]]
*
[[link removed]]
*
*
[[link removed]]
INTERPRET THE WORLD AND CHANGE IT
Submit via web
[[link removed]]
Submit via email
Frequently asked questions
[[link removed]]
Manage subscription
[[link removed]]
Visit xxxxxx.org
[[link removed]]
Twitter [[link removed]]
Facebook [[link removed]]
[link removed]
To unsubscribe, click the following link:
[link removed]
Message Analysis
- Sender: Portside
- Political Party: n/a
- Country: United States
- State/Locality: n/a
- Office: n/a
