Email
New Issue of PxWire: The Latest in HIV Prevention R&D and Delivery
| From | AVAC <[email protected]> |
| Subject | New Issue of PxWire: The Latest in HIV Prevention R&D and Delivery |
| Date | May 22, 2024 3:37 PM |
Links have been removed from this email. Learn more in the FAQ.
Links have been removed from this email. Learn more in the FAQ.
Our quarterly update covering the latest in the field of biomedical HIV prevention research & development.
View this email in your browser ([link removed])
PxWire is AVAC’s quarterly update covering the latest in the field of biomedical HIV prevention research and development, implementation and advocacy. Each issue includes updates, emerging issues and upcoming events. A PDF version of this report is also available.
[link removed] The full issue is available online ([link removed]) as a webpage and PDF ([link removed]) .
------------------------------------------------------------
** Progress in PrEP Uptake
------------------------------------------------------------
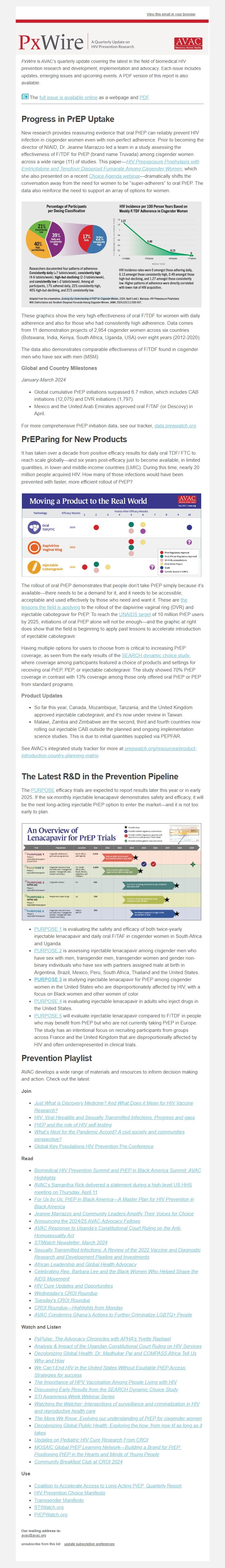
New research provides reassuring evidence that oral PrEP can reliably prevent HIV infection in cisgender women even with non-perfect adherence. Prior to becoming the director of NIAID, Dr. Jeanne Marrazzo led a team in a study assessing the effectiveness of F/TDF for PrEP (brand name Truvada) among cisgender women across a wide range (11) of studies. This paper—HIV Preexposure Prophylaxis with Emtricitabine and Tenofovir Disoproxil Fumarate Among Cisgender Women ([link removed]) , which she also presented on a recent Choice Agenda webinar ([link removed]) —dramatically shifts the conversation away from the need for women to be “super-adherers” to oral PrEP. The data also reinforce the need to support an array of options for women.
These graphics show the very high effectiveness of oral F/TDF for women with daily adherence and also for those who had consistently high adherence. Data comes from 11 demonstration projects of 2,954 cisgender women across six countries (Botswana, India, Kenya, South Africa, Uganda, USA) over eight years (2012-2020).
The data also demonstrates comparable effectiveness of F/TDF found in cisgender men who have sex with men (MSM).
** Global and Country Milestones
------------------------------------------------------------
January-March 2024
* Global cumulative PrEP initiations surpassed 6.7 million, which includes CAB initiations (12,075) and DVR initiations (1,797).
* Mexico and the United Arab Emirates approved oral F/TAF (or Descovy) in April.
For more comprehensive PrEP initiation data, see our tracker, data.prepwatch.org ([link removed]) .
** PrEParing for New Products
------------------------------------------------------------
It has taken over a decade from positive efficacy results for daily oral TDF/ FTC to reach scale globally—and six years post-efficacy just to become available, in limited quantities, in lower-and middle-income countries (LMIC). During this time, nearly 20 million people acquired HIV. How many of those infections would have been prevented with faster, more efficient rollout of PrEP?
The rollout of oral PrEP demonstrates that people don’t take PrEP simply because it’s available—there needs to be a demand for it, and it needs to be accessible, acceptable and used effectively by those who need and want it. These are the lessons the field is applying ([link removed]) to the rollout of the dapivirine vaginal ring (DVR) and injectable cabotegravir for PrEP. To reach the UNAIDS target ([link removed]) of 10 million PrEP users by 2025, initiations of oral PrEP alone will not be enough—and the graphic at right does show that the field is beginning to apply past lessons to accelerate introduction of injectable cabotegravir.
Having multiple options for users to choose from is critical to increasing PrEP coverage, as seen from the early results of the SEARCH dynamic choice study ([link removed]) , where coverage among participants featured a choice of products and settings for receiving oral PrEP, PEP, or injectable cabotegravir. The study showed 70% PrEP coverage in contrast with 13% coverage among those only offered oral PrEP or PEP from standard programs.
** Product Updates
------------------------------------------------------------
* So far this year, Canada, Mozambique, Tanzania, and the United Kingdom approved injectable cabotegravir, and it’s now under review in Taiwan.
* Malawi, Zambia and Zimbabwe are the second, third and fourth countries now rolling out injectable CAB outside the planned and ongoing implementation science studies. This is due to initial quantities supplied via PEPFAR.
See AVAC’s integrated study tracker for more at prepwatch.org/resources/product-introduction-country-planning-matrix ([link removed]) .
** The Latest R&D in the Prevention Pipeline
------------------------------------------------------------
The PURPOSE ([link removed]) efficacy trials are expected to report results later this year or in early 2025. If the six-monthly injectable lenacapavir demonstrates safety and efficacy, it will be the next long-acting injectable PrEP option to enter the market—and it is not too early to plan.
* PURPOSE 1 ([link removed]) is evaluating the safety and efficacy of both twice-yearly injectable lenacapavir and daily oral F/TAF in cisgender women in South Africa and Uganda.
* PURPOSE 2 ([link removed]) is assessing injectable lenacapavir among cisgender men who have sex with men, transgender men, transgender women and gender non-binary individuals who have sex with partners assigned male at birth in Argentina, Brazil, Mexico, Peru, South Africa, Thailand and the United States.
* PURPOSE 3 ([link removed]) is studying injectable lenacapavir for PrEP among cisgender women in the United States who are disproportionately affected by HIV, with a focus on Black women and other women of color.
* PURPOSE 4 ([link removed]) is evaluating injectable lenacapavir in adults who inject drugs in the United States.
* PURPOSE 5 ([link removed]) will evaluate injectable lenacapavir compared to F/TDF in people who may benefit from PrEP but who are not currently taking PrEP in Europe. The study has an intentional focus on recruiting participants from groups across France and the United Kingdom that are disproportionally affected by HIV and often underrepresented in clinical trials.
** Prevention Playlist
------------------------------------------------------------
AVAC develops a wide range of materials and resources to inform decision making and action. Check out the latest:
Join
* Just What is Discovery Medicine? And What Does it Mean for HIV Vaccine Research? ([link removed])
* HIV, Viral Hepatitis and Sexually Transmitted Infections: Progress and gaps ([link removed])
* PrEP and the role of HIV self-testing ([link removed])
* What’s Next for the Pandemic Accord? A civil society and communities perspective? ([link removed])
* Global Key Populations HIV Prevention Pre-Conference ([link removed])
Read
* Biomedical HIV Prevention Summit and PrEP in Black America Summit: AVAC Highlights ([link removed])
* AVAC’s Samantha Rick delivered a statement during a high-level US HHS meeting on Thursday, April 11 ([link removed])
* For Us by Us: PrEP in Black America—A Master Plan for HIV Prevention in Black America ([link removed])
* Jeanne Marrazzo and Community Leaders Amplify Their Voices for Choice ([link removed])
* Announcing the 2024/25 AVAC Advocacy Fellows ([link removed])
* AVAC Response to Uganda’s Constitutional Court Ruling on the Anti-Homosexuality Act ([link removed])
* STIWatch Newsletter, March 2024 ([link removed])
* Sexually Transmitted Infections: A Review of the 2022 Vaccine and Diagnostic Research and Development Pipeline and Investments ([link removed])
* African Leadership and Global Health Advocacy ([link removed])
* Celebrating Rep. Barbara Lee and the Black Women Who Helped Shape the AIDS Movement ([link removed])
* HIV Cure Updates and Opportunities ([link removed])
* Wednesday’s CROI Roundup ([link removed])
* Tuesday’s CROI Roundup ([link removed])
* CROI Roundup—Highlights from Monday ([link removed])
* AVAC Condemns Ghana’s Actions to Further Criminalize LGBTQ+ People ([link removed])
Watch and Listen
* PxPulse: The Advocacy Chronicles with APHA’s Yvette Raphael ([link removed])
* Analysis & Impact of the Ugandan Constitutional Court Ruling on HIV Services ([link removed])
* Decolonizing Global Health: Dr. Madhukar Pai and COMPASS Africa Tell Us Why and How ([link removed])
* We Can’t End HIV in the United States Without Equitable PrEP Access: Strategies for success ([link removed])
* The Importance of HPV Vaccination Among People Living with HIV ([link removed])
* Discussing Early Results from the SEARCH Dynamic Choice Study ([link removed])
* STI Awareness Week Webinar Series ([link removed])
* Watching the Watcher: Intersections of surveillance and criminalization in HIV and reproductive health care ([link removed])
* The More We Know: Evolving our understanding of PrEP for cisgender women ([link removed])
* Decolonizing Global Public Health: Exploring the how, from now til as long as it takes ([link removed])
* Updates on Pediatric HIV Cure Research From CROI ([link removed])
* MOSAIC Global PrEP Learning Network—Building a Brand for PrEP: Positioning PrEP in the Hearts and Minds of Young People ([link removed])
* Community Breakfast Club at CROI 2024 ([link removed])
Use
* Coalition to Accelerate Access to Long-Acting PrEP, Quarterly Report ([link removed])
* HIV Prevention Choice Manifesto ([link removed])
* Transgender Manifesto ([link removed])
* STIWatch.org ([link removed])
* PrEPWatch.org ([link removed])
============================================================
Our mailing address is:
** [email protected] (mailto:[email protected])
** unsubscribe from this list ([link removed])
** update subscription preferences ([link removed])
View this email in your browser ([link removed])
PxWire is AVAC’s quarterly update covering the latest in the field of biomedical HIV prevention research and development, implementation and advocacy. Each issue includes updates, emerging issues and upcoming events. A PDF version of this report is also available.
[link removed] The full issue is available online ([link removed]) as a webpage and PDF ([link removed]) .
------------------------------------------------------------
** Progress in PrEP Uptake
------------------------------------------------------------
New research provides reassuring evidence that oral PrEP can reliably prevent HIV infection in cisgender women even with non-perfect adherence. Prior to becoming the director of NIAID, Dr. Jeanne Marrazzo led a team in a study assessing the effectiveness of F/TDF for PrEP (brand name Truvada) among cisgender women across a wide range (11) of studies. This paper—HIV Preexposure Prophylaxis with Emtricitabine and Tenofovir Disoproxil Fumarate Among Cisgender Women ([link removed]) , which she also presented on a recent Choice Agenda webinar ([link removed]) —dramatically shifts the conversation away from the need for women to be “super-adherers” to oral PrEP. The data also reinforce the need to support an array of options for women.
These graphics show the very high effectiveness of oral F/TDF for women with daily adherence and also for those who had consistently high adherence. Data comes from 11 demonstration projects of 2,954 cisgender women across six countries (Botswana, India, Kenya, South Africa, Uganda, USA) over eight years (2012-2020).
The data also demonstrates comparable effectiveness of F/TDF found in cisgender men who have sex with men (MSM).
** Global and Country Milestones
------------------------------------------------------------
January-March 2024
* Global cumulative PrEP initiations surpassed 6.7 million, which includes CAB initiations (12,075) and DVR initiations (1,797).
* Mexico and the United Arab Emirates approved oral F/TAF (or Descovy) in April.
For more comprehensive PrEP initiation data, see our tracker, data.prepwatch.org ([link removed]) .
** PrEParing for New Products
------------------------------------------------------------
It has taken over a decade from positive efficacy results for daily oral TDF/ FTC to reach scale globally—and six years post-efficacy just to become available, in limited quantities, in lower-and middle-income countries (LMIC). During this time, nearly 20 million people acquired HIV. How many of those infections would have been prevented with faster, more efficient rollout of PrEP?
The rollout of oral PrEP demonstrates that people don’t take PrEP simply because it’s available—there needs to be a demand for it, and it needs to be accessible, acceptable and used effectively by those who need and want it. These are the lessons the field is applying ([link removed]) to the rollout of the dapivirine vaginal ring (DVR) and injectable cabotegravir for PrEP. To reach the UNAIDS target ([link removed]) of 10 million PrEP users by 2025, initiations of oral PrEP alone will not be enough—and the graphic at right does show that the field is beginning to apply past lessons to accelerate introduction of injectable cabotegravir.
Having multiple options for users to choose from is critical to increasing PrEP coverage, as seen from the early results of the SEARCH dynamic choice study ([link removed]) , where coverage among participants featured a choice of products and settings for receiving oral PrEP, PEP, or injectable cabotegravir. The study showed 70% PrEP coverage in contrast with 13% coverage among those only offered oral PrEP or PEP from standard programs.
** Product Updates
------------------------------------------------------------
* So far this year, Canada, Mozambique, Tanzania, and the United Kingdom approved injectable cabotegravir, and it’s now under review in Taiwan.
* Malawi, Zambia and Zimbabwe are the second, third and fourth countries now rolling out injectable CAB outside the planned and ongoing implementation science studies. This is due to initial quantities supplied via PEPFAR.
See AVAC’s integrated study tracker for more at prepwatch.org/resources/product-introduction-country-planning-matrix ([link removed]) .
** The Latest R&D in the Prevention Pipeline
------------------------------------------------------------
The PURPOSE ([link removed]) efficacy trials are expected to report results later this year or in early 2025. If the six-monthly injectable lenacapavir demonstrates safety and efficacy, it will be the next long-acting injectable PrEP option to enter the market—and it is not too early to plan.
* PURPOSE 1 ([link removed]) is evaluating the safety and efficacy of both twice-yearly injectable lenacapavir and daily oral F/TAF in cisgender women in South Africa and Uganda.
* PURPOSE 2 ([link removed]) is assessing injectable lenacapavir among cisgender men who have sex with men, transgender men, transgender women and gender non-binary individuals who have sex with partners assigned male at birth in Argentina, Brazil, Mexico, Peru, South Africa, Thailand and the United States.
* PURPOSE 3 ([link removed]) is studying injectable lenacapavir for PrEP among cisgender women in the United States who are disproportionately affected by HIV, with a focus on Black women and other women of color.
* PURPOSE 4 ([link removed]) is evaluating injectable lenacapavir in adults who inject drugs in the United States.
* PURPOSE 5 ([link removed]) will evaluate injectable lenacapavir compared to F/TDF in people who may benefit from PrEP but who are not currently taking PrEP in Europe. The study has an intentional focus on recruiting participants from groups across France and the United Kingdom that are disproportionally affected by HIV and often underrepresented in clinical trials.
** Prevention Playlist
------------------------------------------------------------
AVAC develops a wide range of materials and resources to inform decision making and action. Check out the latest:
Join
* Just What is Discovery Medicine? And What Does it Mean for HIV Vaccine Research? ([link removed])
* HIV, Viral Hepatitis and Sexually Transmitted Infections: Progress and gaps ([link removed])
* PrEP and the role of HIV self-testing ([link removed])
* What’s Next for the Pandemic Accord? A civil society and communities perspective? ([link removed])
* Global Key Populations HIV Prevention Pre-Conference ([link removed])
Read
* Biomedical HIV Prevention Summit and PrEP in Black America Summit: AVAC Highlights ([link removed])
* AVAC’s Samantha Rick delivered a statement during a high-level US HHS meeting on Thursday, April 11 ([link removed])
* For Us by Us: PrEP in Black America—A Master Plan for HIV Prevention in Black America ([link removed])
* Jeanne Marrazzo and Community Leaders Amplify Their Voices for Choice ([link removed])
* Announcing the 2024/25 AVAC Advocacy Fellows ([link removed])
* AVAC Response to Uganda’s Constitutional Court Ruling on the Anti-Homosexuality Act ([link removed])
* STIWatch Newsletter, March 2024 ([link removed])
* Sexually Transmitted Infections: A Review of the 2022 Vaccine and Diagnostic Research and Development Pipeline and Investments ([link removed])
* African Leadership and Global Health Advocacy ([link removed])
* Celebrating Rep. Barbara Lee and the Black Women Who Helped Shape the AIDS Movement ([link removed])
* HIV Cure Updates and Opportunities ([link removed])
* Wednesday’s CROI Roundup ([link removed])
* Tuesday’s CROI Roundup ([link removed])
* CROI Roundup—Highlights from Monday ([link removed])
* AVAC Condemns Ghana’s Actions to Further Criminalize LGBTQ+ People ([link removed])
Watch and Listen
* PxPulse: The Advocacy Chronicles with APHA’s Yvette Raphael ([link removed])
* Analysis & Impact of the Ugandan Constitutional Court Ruling on HIV Services ([link removed])
* Decolonizing Global Health: Dr. Madhukar Pai and COMPASS Africa Tell Us Why and How ([link removed])
* We Can’t End HIV in the United States Without Equitable PrEP Access: Strategies for success ([link removed])
* The Importance of HPV Vaccination Among People Living with HIV ([link removed])
* Discussing Early Results from the SEARCH Dynamic Choice Study ([link removed])
* STI Awareness Week Webinar Series ([link removed])
* Watching the Watcher: Intersections of surveillance and criminalization in HIV and reproductive health care ([link removed])
* The More We Know: Evolving our understanding of PrEP for cisgender women ([link removed])
* Decolonizing Global Public Health: Exploring the how, from now til as long as it takes ([link removed])
* Updates on Pediatric HIV Cure Research From CROI ([link removed])
* MOSAIC Global PrEP Learning Network—Building a Brand for PrEP: Positioning PrEP in the Hearts and Minds of Young People ([link removed])
* Community Breakfast Club at CROI 2024 ([link removed])
Use
* Coalition to Accelerate Access to Long-Acting PrEP, Quarterly Report ([link removed])
* HIV Prevention Choice Manifesto ([link removed])
* Transgender Manifesto ([link removed])
* STIWatch.org ([link removed])
* PrEPWatch.org ([link removed])
============================================================
Our mailing address is:
** [email protected] (mailto:[email protected])
** unsubscribe from this list ([link removed])
** update subscription preferences ([link removed])
Message Analysis
- Sender: AVAC: Global Advocacy for HIV Prevention
- Political Party: n/a
- Country: n/a
- State/Locality: n/a
- Office: n/a
-
Email Providers:
- MailChimp
