|
FDA and CDC Clear Another Experimental COVID Shot You Shouldn't Get
As predicted, U.S. Health officials signed off on "updated" COVID-19 vaccines with minimal data showing the shots are effective, let alone safe.
I’m not sure how anyone can trust the Biden administration or our U.S. health agencies at this point. Even if they did like the idea of a COVID-19 vaccine, I’m not sure how anyone could render them safe or effective based on the laughable “approval process” these modified-RNA gene therapy products are going through.
Americans have to submit more documentation to renew their driver’s licenses than Pfizer and Moderna are having to turn over on their experimental clot shots, and that, in and of itself, is a problem.
And this year, Americans aren’t just being manipulated into receiving one vaccine, they’re being pushed to receive three vaccines: the new updated COVID booster, the recently approved RSV vaccine, and an ineffective quadrivalent influenza vaccine—many versions of which contain a mercury-based preservative known as thimerosal.
Like myself, you may have already decided that you’re never assaulting your body with these experimental products, or perhaps you’re on the fence because you’re still struggling with the cognitive dissonance that comes with having to wrap your mind around the fact we’ve all been played and are stuck in an abusive relationship with the government.
Let this article remind you that U.S. health agencies learned nothing from their mistakes and atrocities committed during the COVID-19 pandemic—and that they most certainly have not changed.
If they’re willing to bend over for the Biden administration and bow down to the pharmaceutical companies for COVID-19 vaccines, they’re likely doing it with other products as well. (Cough…Ozempic and Wegovy.)
Biden Admin Launches COVID-19, Version 2.0
Only 17% of Americans chose to receive the last COVID-19 bivalent booster, but that didn't stop the Biden administration from relaunching its COVID agenda and sending billions of taxpayer dollars to Big Pharma. Before U.S. regulatory agencies had even signed off on the new shots, the Biden administration said they would be available by mid-September.
In fact, even though only 17% of Americans chose to get “boosted” last fall, the Biden administration upped its orders two weeks ago for the pediatric version of the new COVID vaccines from 14.5 million doses at $1.3 billion to 20 million doses for $1.7 billion.
How do they have the uncanny ability to predict what a U.S. regulatory agency will do? That’s a valid question and one asked by many the last time the Biden administration pressured regulatory agencies and their “independent” vaccine advisors to approve the shots—causing the resignation of two top FDA officials.
But just like the Biden administration predicted, U.S. regulatory agencies let the new shots sail through the approval process this week with next to no data showing they're safe or effective.
The FDA on Sep. 11 authorized new single-strain COVID-19 vaccines by Pfizer and Moderna without adequate clinical data showing the shots are safe or effective.
According to the FDA, the new vaccines are authorized for emergency use for children 6 months through 11 years of age and are fully approved for people 12 and older.
“The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality,” said Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research. “We very much encourage those who are eligible to consider getting vaccinated.”
We should all be questioning the FDA’s “rigorous scientific standards,” which included signing off on Pfizer’s new vaccine with efficacy data from only ten mice. At the same time, maybe we should view two additional mice as an improvement over the eight mice included the last time Pfizer sought to get a new COVID-19 vaccine through the FDA’s revolving door.
The CDC’s vaccine advisors met on Sep. 12 after the FDA’s decision to discuss who should receive an updated vaccine. Some advisors questioned whether there was enough data to approve the vaccine universally for everyone, but only one member, Dr. Pablo Sanchez, voted against the recommendation.
“CDC is now recommending updated COVID-19 vaccination for everyone 6 months and older to better protect you and your loved ones,” CDC director Dr. Mandy Cohen said in a press release after signing off on the panel’s recommendations.
Although the vaccines are considered “updated,” they still target an almost obsolete strain—omicron XXB.1.5 variant, which currently represents only 3.1% of strains.
While it's recognized the new vaccines do not target current dominant variants, vaccine makers who stand to make billions off their products say their vaccines will still offer protection against those strains as children return to school and the weather cools down. How do they know this? They don’t. There's no data to support this assertion whatsoever, and the “fact-checkers” are out to lunch.
Pfizer CEO Albert Bourla said in a press release that he expects the vaccine will be available “in the upcoming days,” which is consistent with the timeline the Biden administration gave before the new shots were even authorized.
Pfizer CEO Albert Bourla and Moderna CEO Stéphane Bancel, who are not medical doctors, are urging Americans to receive their updated COVID shot during the same appointment as their annual flu shot, even though the co-administration of these two vaccines has not been tested for safety.
It’s convenient, though. If a person gets multiple vaccines simultaneously, it’s pretty hard to pinpoint which shot caused an adverse event. It’s also great for marketing.
In fact, Moderna recently told investors it was focused on increasing vaccine sales through “awareness campaigns” that will “connect COVID-19 with seasonal flu vaccines to drive consumers to get vaccinated this fall.”
Critics Criticize Lack of Human Clinical Trials
Critics emphasized the lack of human clinical trials before approving the injections, as Pfizer and Moderna shots have only been tested on mice—not people.
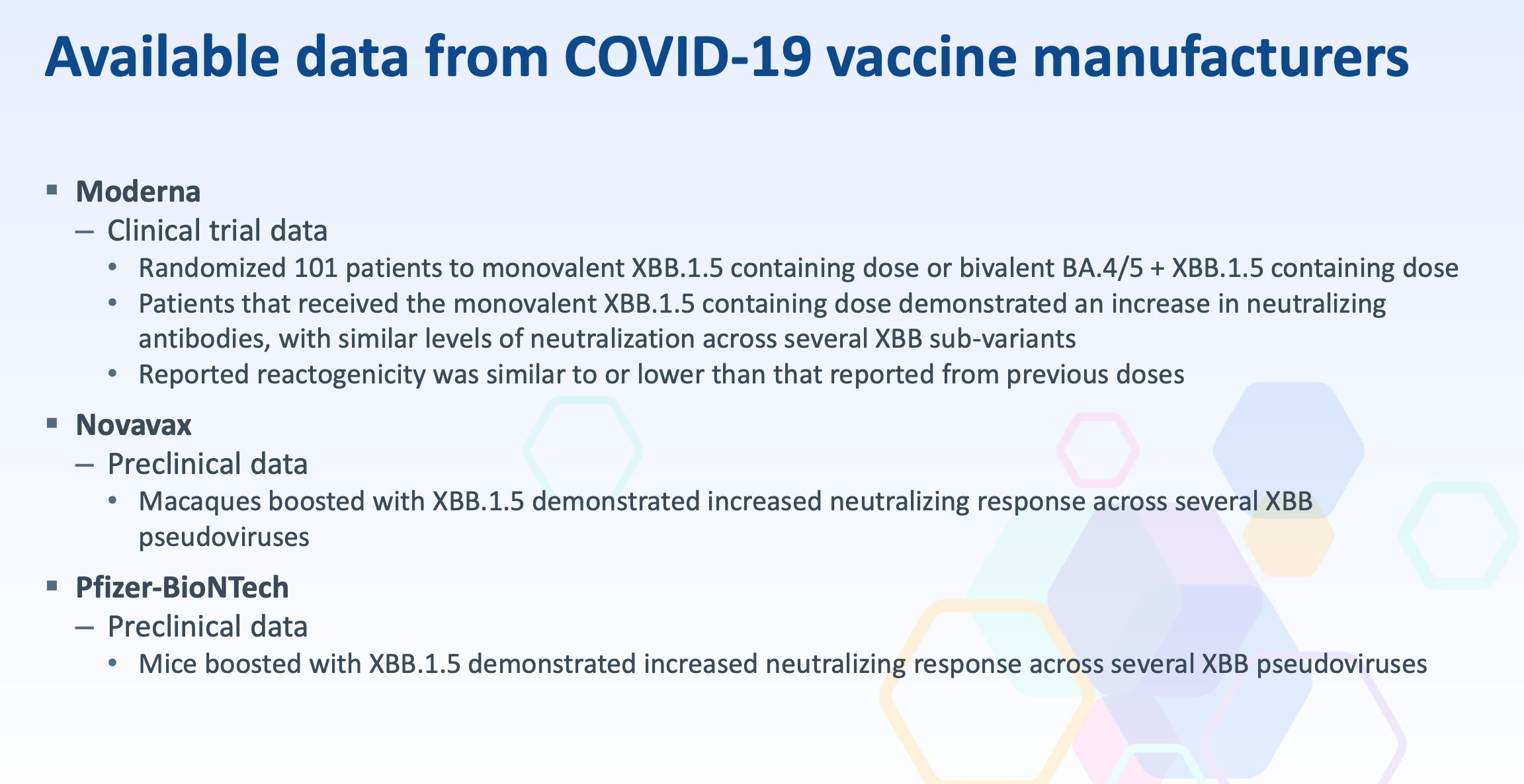
Here’s the available data on COVID-19 vaccines presented by manufacturers Moderna, Pfizer, and Novavax:
For Moderna, there is clinical data on just 101 patients—half of whom received the new monovalent vaccine and half who received the bivalent vaccine. Per the data, no individuals received a placebo, which is essential for a proper risk-benefit analysis.
For Pfizer, there is preclinical data from 10 mice. Pfizer data on efficacy or adverse events is entirely unknown.
Although an updated Novavax vaccine has not yet been authorized, it appears their preclinical data isn’t much different.
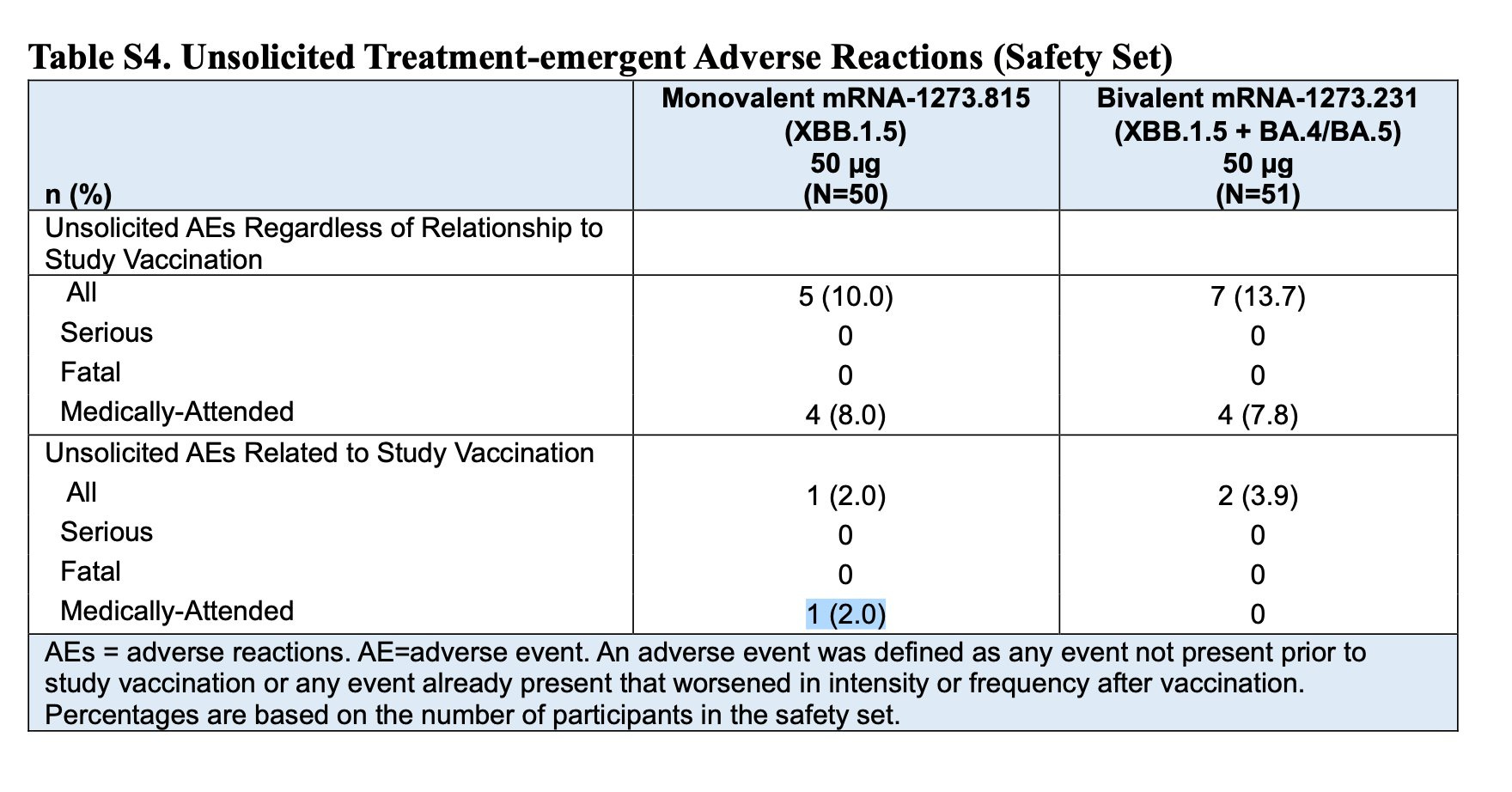
Of the 50 study participants who received Moderna’s new monovalent vaccine, 1 in 50 people had a medically-attended adverse event related to the vaccine, yet the CDC said with certainty that the vaccine’s benefits outweigh the risks for all individuals.
It’s important to note this trial began four months ago, yet Moderna only reported 14-day side effects. Why is that?
“What if I told you 1 in 50 people who took a new medication had a ‘medically-attended adverse event,’ and the manufacturer refused to disclose what the complication was, would you take it,” Dr. Marty Makary, physician and professor at Johns Hopkins posted on “X.”
“And what if the theoretical benefit only lasted for a 3-mo window, after which time the benefit is gone? And that the FDA cleared it without any human outcomes data, would you take it? And that European regulators are not universally recommending the same medication for everyone as the CDC is?
“And that Drs. Ashish Jha and Mandy Cohen are making unsupported claims that it reduces hospitalizations long-COVID and makes you less likely to spread COVID (if the manufacturers made those same claims, they could be fined by the FDA for making false marketing claims beyond its approved indication).
“That’s the new Moderna COVID vaccine. FDA or Moderna (I can’t tell the difference sometimes) should disclose the details of the clinical trial complication rather than keeping it secret.”
The only “study” we have from Moderna is this pre-print published Sep. 7 on MedRxiv, and the data it provides is exclusive to antibody production. Although they state they have vaccine efficacy data, the CDC and FDA have not published or requested it.
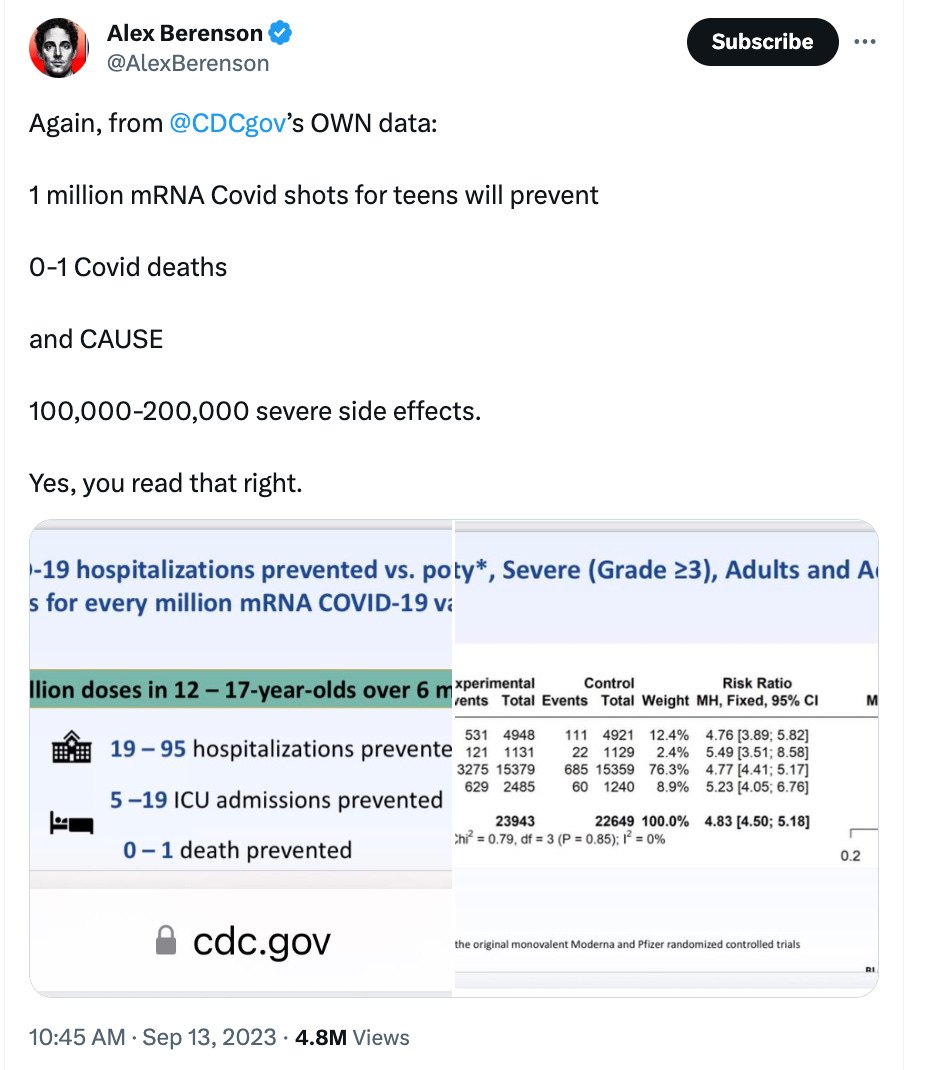
Journalist Alex Berenson looked at the CDC’s COVID-19 vaccine data and pointed out that 1 million mRNA COVID vaccines for teens will prevent 0 to 1 COVID-19 deaths while causing 100,000-200,000 severe side effects.
“The math: Moderna trial teen subjects had a 25% risk of Grade 3/4 adverse events if they received the shot, 5% for placebo,” Berenson posted on “X.”
“For Pfizer, the rates were about 11% and 2% (Pfizer is a lower dose; why the placebo rates were different, IDK.) That’s a gap of 0.09 to 0.2, x1MM shots,” Berenson said.
“Pushing a new COVID vaccine without human-outcomes data makes a mockery of the scientific method and our regulatory process. In fact, why have an FDA if White House doctors can simply declare a drug to be safe after discussing secret data in private meetings with pharma,” wrote Makary and epidemiologist Dr. Tracy Høeg in The New York Post.
“If public health officials don’t want a repeat disappointing turnout of Americans who get the COVID booster shot, they should require a proper clinical trial to show the American people the benefit,” they added. “Public health leaders cannot afford to squander any more credibility and money on interventions with no scientific support.”
The bottom line? If you feel you need a vaccine, perhaps it would be best to get vaccinated against the government’s propaganda because these aren’t “vaccines,” they have not been proven safe or effective, and they do not work—unless, of course, your goal is to increase your chances of getting an autoimmune condition, neurological damage, a heart problem, or a blood clot.
You’re currently a free subscriber to Megan Redshaw's Newsletter. Upgrade your subscription to get the full experience and support Megan’s work.