| From | xxxxxx <[email protected]> |
| Subject | Sunday Science: Aging Is Complicated |
| Date | July 10, 2023 12:10 AM |
Links have been removed from this email. Learn more in the FAQ.
Links have been removed from this email. Learn more in the FAQ.
[A biologist explains why no two people or cells age the same way,
and what this means for anti-aging interventions]
[[link removed]]
SUNDAY SCIENCE: AGING IS COMPLICATED
[[link removed]]
Ellen Quarles
July 6, 2023
The Conversation
[[link removed]]
*
[[link removed]]
*
[[link removed]]
*
*
[[link removed]]
_ A biologist explains why no two people or cells age the same way,
and what this means for anti-aging interventions _
>While some people may be older in chronological age, their
biological age might be much younger., FangXiaNuo/E+ via Getty Images
You likely know someone who seems to age slowly
[[link removed]],
appearing years younger than their birth date suggests. And you likely
have seen the opposite – someone whose body and mind seem much more
ravaged by time than others. Why do some people seem to glide though
their golden years and others physiologically struggle in midlife?
I have worked in the field of aging
[[link removed]] for all
of my scientific career, and I teach the cellular and molecular
biology of aging at the University of Michigan. Aging research
doesn’t tend to be about finding the one cure that fixes all that
may ail you in old age. Instead, the last decade or two of work points
to aging as a multi-factoral process – and no single intervention
can stop it all.
What is aging?
There are many different definitions of aging, but scientists
generally agree upon some common features
[[link removed]]: Aging is a
time-dependent process that results in increased vulnerability to
disease, injury and death. This process is both intrinsic, when your
own body causes new problems, and extrinsic, when environmental
insults damage your tissues.
Your body is comprised of trillions of cells
[[link removed]], and
each one is not only responsible for one or more functions specific to
the tissue it resides in, but must also do all the work of keeping
itself alive. This includes metabolizing nutrients, getting rid of
waste, exchanging signals with other cells and adapting to stress.
Aging results from a number of physiological factors.
The trouble is that every single process and component in each of your
cells can be interrupted or damaged
[[link removed]]. So your cells spend a
lot of energy each day preventing, recognizing and fixing those
problems.
Aging can be thought of as a gradual loss of the ability to maintain
homeostasis [[link removed]] – a state of balance
among body systems – either by not being able to prevent or
recognize damage and poor function, or by not adequately or rapidly
fixing problems as they occur. Aging results from a combination of
these issues. Decades of research has shown that nearly every cellular
process becomes more impaired with age.
Repairing DNA and recycling proteins
Most research on cellular aging focuses on studying how DNA and
proteins change with age. Scientists are also beginning to address the
potential roles many other important biomolecules in the cell play in
aging as well.
One of the cell’s chief jobs is to maintain its DNA – the
instruction manual a cell’s machinery reads to produce specific
proteins. DNA maintenance involves protecting against, and accurately
repairing, damage to genetic material and the molecules binding to it.
Proteins are the workers of the cell. They perform chemical reactions,
provide structural support, send and receive messages, hold and
release energy, and much more. If the protein is damaged, the cell
uses mechanisms involving [[link removed]]
special proteins
[[link removed]]
that either attempt to fix the broken protein or send it off for
recycling. Similar mechanisms tuck proteins out of the way or destroy
them when they are no longer needed. That way, its components can be
used later to build a new protein.
Aging disrupts a delicate biological network
The cross-talk between the components inside cells, cells as a whole,
organs and the environment is a complex and ever-changing network of
information.
When all processes involved in creating and maintaining DNA and
protein function are working normally, the different compartments
within a cell serving specialized roles – called organelles
[[link removed]] – can maintain
the cell’s health and function. For an organ to work well, the
majority of the cells that make it up need to function well. And for a
whole organism to survive and thrive, all of the organs in its body
need to work well.
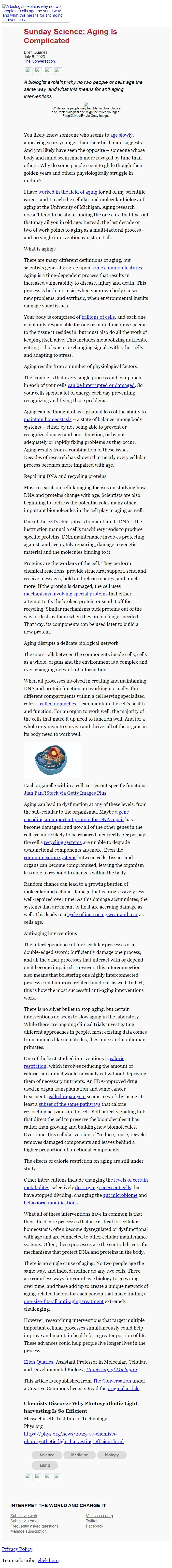
[Illustration of cross-section of an animal cell and its organelles]
[[link removed]]
Each organelle within a cell carries out specific functions. Jian
Fan/iStock via Getty Images Plus
[[link removed]]
Aging can lead to dysfunction at any of these levels, from the
sub-cellular to the organismal. Maybe a gene encoding an important
protein for DNA repair [[link removed]] has
become damaged, and now all of the other genes in the cell are more
likely to be repaired incorrectly. Or perhaps the cell’s recycling
systems
[[link removed]]
are unable to degrade dysfunctional components anymore. Even the
communication systems
[[link removed](18)30026-3] between cells,
tissues and organs can become compromised, leaving the organism less
able to respond to changes within the body.
Random chance can lead to a growing burden of molecular and cellular
damage that is progressively less well-repaired over time. As this
damage accumulates, the systems that are meant to fix it are accruing
damage as well. This leads to a cycle of increasing wear and tear
[[link removed]] as cells age.
Anti-aging interventions
The interdependence of life’s cellular processes is a double-edged
sword: Sufficiently damage one process, and all the other processes
that interact with or depend on it become impaired. However, this
interconnection also means that bolstering one highly interconnected
process could improve related functions as well. In fact, this is how
the most successful anti-aging interventions work.
There is no silver bullet to stop aging, but certain interventions do
seem to slow aging in the laboratory. While there are ongoing clinical
trials investigating different approaches in people, most existing
data comes from animals like nematodes, flies, mice and nonhuman
primates.
One of the best studied interventions is caloric restriction
[[link removed]], which involves reducing the
amount of calories an animal would normally eat without depriving them
of necessary nutrients. An FDA-approved drug used in organ
transplantation and some cancer treatments called rapamycin
[[link removed]] seems to work by using at
least a subset of the same pathways
[[link removed]] that calorie restriction
activates in the cell. Both affect signaling hubs that direct the cell
to preserve the biomolecules it has rather than growing and building
new biomolecules. Over time, this cellular version of “reduce,
reuse, recycle” removes damaged components and leaves behind a
higher proportion of functional components.
The effects of calorie restriction on aging are still under study.
Other interventions include changing the levels of certain metabolites
[[link removed]],
selectively destroying senescent cells
[[link removed]] that have stopped dividing,
changing the gut microbiome
[[link removed]]
and behavioral modifications [[link removed]].
What all of these interventions have in common is that they affect
core processes that are critical for cellular homeostasis, often
become dysregulated or dysfunctional with age and are connected to
other cellular maintenance systems. Often, these processes are the
central drivers for mechanisms that protect DNA and proteins in the
body.
There is no single cause of aging. No two people age the same way, and
indeed, neither do any two cells. There are countless ways for your
basic biology to go wrong over time, and these add up to create a
unique network of aging-related factors for each person that make
finding a one-size-fits-all anti-aging treatment
[[link removed]]
extremely challenging.
However, researching interventions that target multiple important
cellular processes simultaneously could help improve and maintain
health for a greater portion of life. These advances could help people
live longer lives in the process.[The Conversation]
Ellen Quarles
[[link removed]],
Assistant Professor in Molecular, Cellular, and Developmental Biology,
_University of Michigan
[[link removed]]_
This article is republished from The Conversation
[[link removed]] under a Creative Commons license. Read
the original article
[[link removed]].
CHEMISTS DISCOVER WHY PHOTOSYNTHETIC LIGHT-HARVESTING IS SO EFFICIENT
Massachusetts Institute of Technology
Phys.org
[link removed]
* Science
[[link removed]]
* Medicine
[[link removed]]
* biology
[[link removed]]
* aging
[[link removed]]
*
[[link removed]]
*
[[link removed]]
*
*
[[link removed]]
INTERPRET THE WORLD AND CHANGE IT
Submit via web
[[link removed]]
Submit via email
Frequently asked questions
[[link removed]]
Manage subscription
[[link removed]]
Visit xxxxxx.org
[[link removed]]
Twitter [[link removed]]
Facebook [[link removed]]
[link removed]
To unsubscribe, click the following link:
[link removed]
and what this means for anti-aging interventions]
[[link removed]]
SUNDAY SCIENCE: AGING IS COMPLICATED
[[link removed]]
Ellen Quarles
July 6, 2023
The Conversation
[[link removed]]
*
[[link removed]]
*
[[link removed]]
*
*
[[link removed]]
_ A biologist explains why no two people or cells age the same way,
and what this means for anti-aging interventions _
>While some people may be older in chronological age, their
biological age might be much younger., FangXiaNuo/E+ via Getty Images
You likely know someone who seems to age slowly
[[link removed]],
appearing years younger than their birth date suggests. And you likely
have seen the opposite – someone whose body and mind seem much more
ravaged by time than others. Why do some people seem to glide though
their golden years and others physiologically struggle in midlife?
I have worked in the field of aging
[[link removed]] for all
of my scientific career, and I teach the cellular and molecular
biology of aging at the University of Michigan. Aging research
doesn’t tend to be about finding the one cure that fixes all that
may ail you in old age. Instead, the last decade or two of work points
to aging as a multi-factoral process – and no single intervention
can stop it all.
What is aging?
There are many different definitions of aging, but scientists
generally agree upon some common features
[[link removed]]: Aging is a
time-dependent process that results in increased vulnerability to
disease, injury and death. This process is both intrinsic, when your
own body causes new problems, and extrinsic, when environmental
insults damage your tissues.
Your body is comprised of trillions of cells
[[link removed]], and
each one is not only responsible for one or more functions specific to
the tissue it resides in, but must also do all the work of keeping
itself alive. This includes metabolizing nutrients, getting rid of
waste, exchanging signals with other cells and adapting to stress.
Aging results from a number of physiological factors.
The trouble is that every single process and component in each of your
cells can be interrupted or damaged
[[link removed]]. So your cells spend a
lot of energy each day preventing, recognizing and fixing those
problems.
Aging can be thought of as a gradual loss of the ability to maintain
homeostasis [[link removed]] – a state of balance
among body systems – either by not being able to prevent or
recognize damage and poor function, or by not adequately or rapidly
fixing problems as they occur. Aging results from a combination of
these issues. Decades of research has shown that nearly every cellular
process becomes more impaired with age.
Repairing DNA and recycling proteins
Most research on cellular aging focuses on studying how DNA and
proteins change with age. Scientists are also beginning to address the
potential roles many other important biomolecules in the cell play in
aging as well.
One of the cell’s chief jobs is to maintain its DNA – the
instruction manual a cell’s machinery reads to produce specific
proteins. DNA maintenance involves protecting against, and accurately
repairing, damage to genetic material and the molecules binding to it.
Proteins are the workers of the cell. They perform chemical reactions,
provide structural support, send and receive messages, hold and
release energy, and much more. If the protein is damaged, the cell
uses mechanisms involving [[link removed]]
special proteins
[[link removed]]
that either attempt to fix the broken protein or send it off for
recycling. Similar mechanisms tuck proteins out of the way or destroy
them when they are no longer needed. That way, its components can be
used later to build a new protein.
Aging disrupts a delicate biological network
The cross-talk between the components inside cells, cells as a whole,
organs and the environment is a complex and ever-changing network of
information.
When all processes involved in creating and maintaining DNA and
protein function are working normally, the different compartments
within a cell serving specialized roles – called organelles
[[link removed]] – can maintain
the cell’s health and function. For an organ to work well, the
majority of the cells that make it up need to function well. And for a
whole organism to survive and thrive, all of the organs in its body
need to work well.
[Illustration of cross-section of an animal cell and its organelles]
[[link removed]]
Each organelle within a cell carries out specific functions. Jian
Fan/iStock via Getty Images Plus
[[link removed]]
Aging can lead to dysfunction at any of these levels, from the
sub-cellular to the organismal. Maybe a gene encoding an important
protein for DNA repair [[link removed]] has
become damaged, and now all of the other genes in the cell are more
likely to be repaired incorrectly. Or perhaps the cell’s recycling
systems
[[link removed]]
are unable to degrade dysfunctional components anymore. Even the
communication systems
[[link removed](18)30026-3] between cells,
tissues and organs can become compromised, leaving the organism less
able to respond to changes within the body.
Random chance can lead to a growing burden of molecular and cellular
damage that is progressively less well-repaired over time. As this
damage accumulates, the systems that are meant to fix it are accruing
damage as well. This leads to a cycle of increasing wear and tear
[[link removed]] as cells age.
Anti-aging interventions
The interdependence of life’s cellular processes is a double-edged
sword: Sufficiently damage one process, and all the other processes
that interact with or depend on it become impaired. However, this
interconnection also means that bolstering one highly interconnected
process could improve related functions as well. In fact, this is how
the most successful anti-aging interventions work.
There is no silver bullet to stop aging, but certain interventions do
seem to slow aging in the laboratory. While there are ongoing clinical
trials investigating different approaches in people, most existing
data comes from animals like nematodes, flies, mice and nonhuman
primates.
One of the best studied interventions is caloric restriction
[[link removed]], which involves reducing the
amount of calories an animal would normally eat without depriving them
of necessary nutrients. An FDA-approved drug used in organ
transplantation and some cancer treatments called rapamycin
[[link removed]] seems to work by using at
least a subset of the same pathways
[[link removed]] that calorie restriction
activates in the cell. Both affect signaling hubs that direct the cell
to preserve the biomolecules it has rather than growing and building
new biomolecules. Over time, this cellular version of “reduce,
reuse, recycle” removes damaged components and leaves behind a
higher proportion of functional components.
The effects of calorie restriction on aging are still under study.
Other interventions include changing the levels of certain metabolites
[[link removed]],
selectively destroying senescent cells
[[link removed]] that have stopped dividing,
changing the gut microbiome
[[link removed]]
and behavioral modifications [[link removed]].
What all of these interventions have in common is that they affect
core processes that are critical for cellular homeostasis, often
become dysregulated or dysfunctional with age and are connected to
other cellular maintenance systems. Often, these processes are the
central drivers for mechanisms that protect DNA and proteins in the
body.
There is no single cause of aging. No two people age the same way, and
indeed, neither do any two cells. There are countless ways for your
basic biology to go wrong over time, and these add up to create a
unique network of aging-related factors for each person that make
finding a one-size-fits-all anti-aging treatment
[[link removed]]
extremely challenging.
However, researching interventions that target multiple important
cellular processes simultaneously could help improve and maintain
health for a greater portion of life. These advances could help people
live longer lives in the process.[The Conversation]
Ellen Quarles
[[link removed]],
Assistant Professor in Molecular, Cellular, and Developmental Biology,
_University of Michigan
[[link removed]]_
This article is republished from The Conversation
[[link removed]] under a Creative Commons license. Read
the original article
[[link removed]].
CHEMISTS DISCOVER WHY PHOTOSYNTHETIC LIGHT-HARVESTING IS SO EFFICIENT
Massachusetts Institute of Technology
Phys.org
[link removed]
* Science
[[link removed]]
* Medicine
[[link removed]]
* biology
[[link removed]]
* aging
[[link removed]]
*
[[link removed]]
*
[[link removed]]
*
*
[[link removed]]
INTERPRET THE WORLD AND CHANGE IT
Submit via web
[[link removed]]
Submit via email
Frequently asked questions
[[link removed]]
Manage subscription
[[link removed]]
Visit xxxxxx.org
[[link removed]]
Twitter [[link removed]]
Facebook [[link removed]]
[link removed]
To unsubscribe, click the following link:
[link removed]
Message Analysis
- Sender: Portside
- Political Party: n/a
- Country: United States
- State/Locality: n/a
- Office: n/a
-
Email Providers:
- L-Soft LISTSERV
