| From | ADEA <[email protected]> |
| Subject | ADEA Advocate - May 9, 2023 |
| Date | May 9, 2023 7:03 PM |
Links have been removed from this email. Learn more in the FAQ.
Links have been removed from this email. Learn more in the FAQ.
View this email in your browser [ [link removed] ] .
American Dental Education Association
Volume 2, No. 98, May 9, 2023
DEA Issues Statement on Temporarily Continuing PHE Telehealth Prescribing Flexibilities
The Drug Enforcement Administration (DEA) issued [ [link removed] ] a statement from Administrator Anne Milgram on COVID-19 telehealth flexibilities for prescribing controlled substances. The DEA decided to temporarily extend the COVID-19 telehealth flexibilities beyond the May 11 end date for the Public Health Emergency (PHE) so that they have more time to “. . . work to find a way forward to give Americans that access [to telehealth prescribing] with appropriate safeguards.”
This decision comes after receiving 38,000 comments in response to two proposed rules (Telemedicine Prescribing of Controlled Substances When the Practitioner and the Patient Have Not Had a Prior In-Person Medical Evaluation [ [link removed] ] and Expansion of Induction of Buprenorphine via Telemedicine Encounter [ [link removed] ] ), which addressed the prescribing of controlled substances based on a telehealth encounter after the conclusion of the PHE. Advocates complained that the DEA’s proposals were “more restrictive than is warranted” and would hinder access to care, particularly for individuals with substance use disorder who started treatment during the PHE.
The proposed regulations would allow for prescribing of a 30-day supply of “Schedule III-V non-narcotic controlled medications” and “buprenorphine for the treatment of opioid use disorder” via telehealth with no prior in-person exam. However, in order to receive more than a 30-day supply, patients who are receiving prescriptions for non-narcotic controlled medications from a provider they have never seen in person will need to have at least one in-person appointment after the public health emergency ends in order to continue receiving their prescriptions.
The temporary rule has not been released yet. However, the DEA, in conjunction with the Department of Health and Human Services, have submitted a draft of the “Temporary Extension of COVID-19 Telemedicine Flexibilities for Prescription of Controlled Medications” to the Office of Management and Budget for final review prior to publication in the Federal Register.
CMS Proposes New Standards to Aid Access to Health Care in Medicaid and CHIP Programs
The Centers for Medicare & Medicaid Services (CMS) proposed two Notices of Proposed Rulemakings (NPRMs). Together, the Ensuring Access to Medicaid Services (Access NPRM) [ [link removed] ] and Medicaid and Children’s Health Insurance Program (CHIP) [ [link removed] ] Managed Care Access, Finance, and Quality (Managed Care NPRM) would establish national standards for access to care for both managed care plans or fee-for-services paid for directly by the states. Specifically, these NPRMs would:
• Establish maximum standards for appointment wait times for Medicaid and Children Health Insurance Program (CHIP) programs.
• Require states to conduct independent secret shopper surveys of Medicaid or CHIP programs to verify compliance with appointment wait time standards and to identify where provider directories are inaccurate.
• Create new payment transparency requirements for states by requiring disclosure of provider payment rates in both fee-for-service and managed care, with the goal of greater insight into how Medicaid payment levels affect access to care.
The comment period for both NPRMs is July 3, 2023.
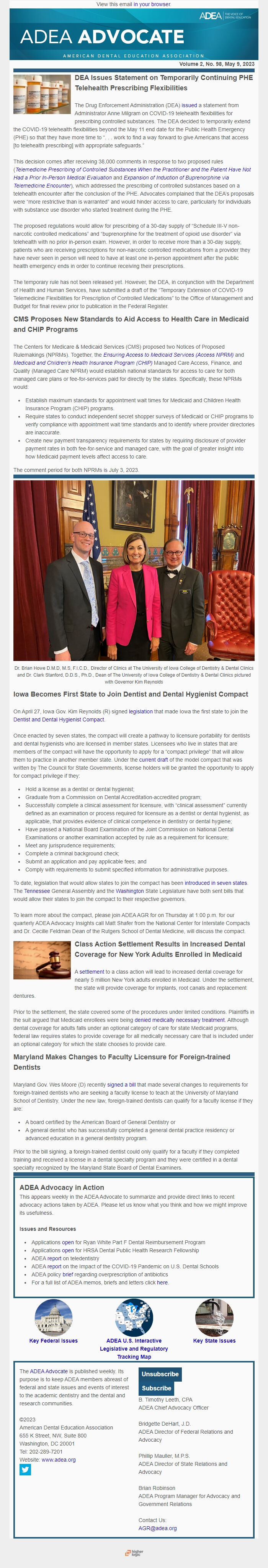
Dr. Brian Howe D.M.D, M.S, F.I.C.D., Director of Clinics at The University of Iowa College of Dentistry & Dental Clinics and Dr. Clark Stanford, D.D.S., Ph.D., Dean of The University of Iowa College of Dentistry & Dental Clinics pictured with Governor Kim Reynolds
Iowa Becomes First State to Join Dentist and Dental Hygienist Compact
On April 27, Iowa Gov. Kim Reynolds (R) signed legislation [ [link removed] ] that made Iowa the first state to join the Dentist and Dental Hygienist Compact [ [link removed] ] .
Once enacted by seven states, the compact will create a pathway to licensure portability for dentists and dental hygienists who are licensed in member states. Licensees who live in states that are members of the compact will have the opportunity to apply for a “compact privilege” that will allow them to practice in another member state. Under the current draft [ [link removed] ] of the model compact that was written by The Council for State Governments, license holders will be granted the opportunity to apply for compact privilege if they:
• Hold a license as a dentist or dental hygienist;
• Graduate from a Commission on Dental Accreditation-accredited program;
• Successfully complete a clinical assessment for licensure, with “clinical assessment” currently defined as an examination or process required for licensure as a dentist or dental hygienist, as applicable, that provides evidence of clinical competence in dentistry or dental hygiene;
• Have passed a National Board Examination of the Joint Commission on National Dental Examinations or another examination accepted by rule as a requirement for licensure;
• Meet any jurisprudence requirements;
• Complete a criminal background check;
• Submit an application and pay applicable fees; and
• Comply with requirements to submit specified information for administrative purposes.
To date, legislation that would allow states to join the compact has been introduced in seven states [ [link removed] ] . The Tennessee [ [link removed] ] General Assembly and the Washington [ [link removed] ] State Legislature have both sent bills that would allow their states to join the compact to their respective governors.
To learn more about the compact, please join ADEA AGR for on Thursday at 1:00 p.m. for our quarterly ADEA Advocacy Insights call Matt Shafer from the National Center for Interstate Compacts and Dr. Cecille Feldman Dean of the Rutgers School of Dental Medicine, will discuss the compact.
Class Action Settlement Results in Increased Dental Coverage for New York Adults Enrolled in Medicaid
A settlement [ [link removed] ] to a class action will lead to increased dental coverage for nearly 5 million New York adults enrolled in Medicaid. Under the settlement, the state will provide coverage for implants, root canals and replacement dentures.
Prior to the settlement, the state covered some of the procedures under limited conditions. Plaintiffs in the suit argued that Medicaid enrollees were being denied medically necessary treatment [ [link removed] ] . Although dental coverage for adults falls under an optional category of care for state Medicaid programs, federal law requires states to provide coverage for all medically necessary care that is included under an optional category for which the state chooses to provide care.
Maryland Makes Changes to Faculty Licensure for Foreign-trained Dentists
Maryland Gov. Wes Moore (D) recently signed a bill [ [link removed] ] that made several changes to requirements for foreign-trained dentists who are seeking a faculty license to teach at the University of Maryland School of Dentistry. Under the new law, foreign-trained dentists can qualify for a faculty license if they are:
• A board certified by the American Board of General Dentistry or
• A general dentist who has successfully completed a general dental practice residency or advanced education in a general dentistry program.
Prior to the bill signing, a foreign-trained dentist could only qualify for a faculty if they completed training and received a license in a dental specialty program and they were certified in a dental specialty recognized by the Maryland State Board of Dental Examiners.
ADEA Advocacy in Action
This appears weekly in the ADEA Advocate to summarize and provide direct links to recent advocacy actions taken by ADEA. Please let us know what you think and how we might improve its usefulness.
Issues and Resources
• Applications open [ [link removed] ] for Ryan White Part F Dental Reimbursement Program
• Applications open [ [link removed] ] for HRSA Dental Public Health Research Fellowship
• ADEA report [ [link removed] ] on teledentistry
• ADEA report [ [link removed] ] on the Impact of the COVID-19 Pandemic on U.S. Dental Schools
• ADEA policy brief [ [link removed] ] regarding overprescription of antibiotics
• For a full list of ADEA memos, briefs and letters click here [ [link removed] ] .
Key Federal Issues [ [link removed] ]
ADEA U.S. Interactive Legislative and Regulatory Tracking Map [ [link removed] ]
Key State Issues [ [link removed] ]
The ADEA Advocate [ [link removed] ] is published weekly. Its purpose is to keep ADEA members abreast of federal and state issues and events of interest to the academic dentistry and the dental and research communities.
©2023
American Dental Education Association
655 K Street, NW, Suite 800
Washington, DC 20001
Tel: 202-289-7201
Website: www.adea.org [ [link removed] ]
twitter
[[link removed]]
Unsubscribe
[link removed]
Subscribe
[link removed][0]&p_colname=p_last_nm&p_varname=p_val_arr[1]&p_colname=p_alias&p_varname=p_val_arr[2]&p_colname=p_login_id&p_varname=p_val_arr[3]&p_colname=p_passwd&p_context=NEWSLETTER&p_success_url=censsaindprofile.section_update%3Fp_profile_ty%3DINDIVIDUAL_PROFILE%26p_skip_confirm_fl%3DY%26p_section_nm%3DNewsletters%26p_format%3D110%26p_msg_txt%3D%26p_cust_id%3D%26p_referrer%3D
B. Timothy Leeth, CPA
ADEA Chief Advocacy Officer
Bridgette DeHart, J.D.
ADEA Director of Federal Relations and Advocacy
Phillip Mauller, M.P.S.
ADEA Director of State Relations and Advocacy
Brian Robinson
ADEA Program Manager for Advocacy and Government Relations
Contact Us:
[email protected] [ [link removed] ]
Powered by Higher Logic [link removed]
American Dental Education Association
Volume 2, No. 98, May 9, 2023
DEA Issues Statement on Temporarily Continuing PHE Telehealth Prescribing Flexibilities
The Drug Enforcement Administration (DEA) issued [ [link removed] ] a statement from Administrator Anne Milgram on COVID-19 telehealth flexibilities for prescribing controlled substances. The DEA decided to temporarily extend the COVID-19 telehealth flexibilities beyond the May 11 end date for the Public Health Emergency (PHE) so that they have more time to “. . . work to find a way forward to give Americans that access [to telehealth prescribing] with appropriate safeguards.”
This decision comes after receiving 38,000 comments in response to two proposed rules (Telemedicine Prescribing of Controlled Substances When the Practitioner and the Patient Have Not Had a Prior In-Person Medical Evaluation [ [link removed] ] and Expansion of Induction of Buprenorphine via Telemedicine Encounter [ [link removed] ] ), which addressed the prescribing of controlled substances based on a telehealth encounter after the conclusion of the PHE. Advocates complained that the DEA’s proposals were “more restrictive than is warranted” and would hinder access to care, particularly for individuals with substance use disorder who started treatment during the PHE.
The proposed regulations would allow for prescribing of a 30-day supply of “Schedule III-V non-narcotic controlled medications” and “buprenorphine for the treatment of opioid use disorder” via telehealth with no prior in-person exam. However, in order to receive more than a 30-day supply, patients who are receiving prescriptions for non-narcotic controlled medications from a provider they have never seen in person will need to have at least one in-person appointment after the public health emergency ends in order to continue receiving their prescriptions.
The temporary rule has not been released yet. However, the DEA, in conjunction with the Department of Health and Human Services, have submitted a draft of the “Temporary Extension of COVID-19 Telemedicine Flexibilities for Prescription of Controlled Medications” to the Office of Management and Budget for final review prior to publication in the Federal Register.
CMS Proposes New Standards to Aid Access to Health Care in Medicaid and CHIP Programs
The Centers for Medicare & Medicaid Services (CMS) proposed two Notices of Proposed Rulemakings (NPRMs). Together, the Ensuring Access to Medicaid Services (Access NPRM) [ [link removed] ] and Medicaid and Children’s Health Insurance Program (CHIP) [ [link removed] ] Managed Care Access, Finance, and Quality (Managed Care NPRM) would establish national standards for access to care for both managed care plans or fee-for-services paid for directly by the states. Specifically, these NPRMs would:
• Establish maximum standards for appointment wait times for Medicaid and Children Health Insurance Program (CHIP) programs.
• Require states to conduct independent secret shopper surveys of Medicaid or CHIP programs to verify compliance with appointment wait time standards and to identify where provider directories are inaccurate.
• Create new payment transparency requirements for states by requiring disclosure of provider payment rates in both fee-for-service and managed care, with the goal of greater insight into how Medicaid payment levels affect access to care.
The comment period for both NPRMs is July 3, 2023.
Dr. Brian Howe D.M.D, M.S, F.I.C.D., Director of Clinics at The University of Iowa College of Dentistry & Dental Clinics and Dr. Clark Stanford, D.D.S., Ph.D., Dean of The University of Iowa College of Dentistry & Dental Clinics pictured with Governor Kim Reynolds
Iowa Becomes First State to Join Dentist and Dental Hygienist Compact
On April 27, Iowa Gov. Kim Reynolds (R) signed legislation [ [link removed] ] that made Iowa the first state to join the Dentist and Dental Hygienist Compact [ [link removed] ] .
Once enacted by seven states, the compact will create a pathway to licensure portability for dentists and dental hygienists who are licensed in member states. Licensees who live in states that are members of the compact will have the opportunity to apply for a “compact privilege” that will allow them to practice in another member state. Under the current draft [ [link removed] ] of the model compact that was written by The Council for State Governments, license holders will be granted the opportunity to apply for compact privilege if they:
• Hold a license as a dentist or dental hygienist;
• Graduate from a Commission on Dental Accreditation-accredited program;
• Successfully complete a clinical assessment for licensure, with “clinical assessment” currently defined as an examination or process required for licensure as a dentist or dental hygienist, as applicable, that provides evidence of clinical competence in dentistry or dental hygiene;
• Have passed a National Board Examination of the Joint Commission on National Dental Examinations or another examination accepted by rule as a requirement for licensure;
• Meet any jurisprudence requirements;
• Complete a criminal background check;
• Submit an application and pay applicable fees; and
• Comply with requirements to submit specified information for administrative purposes.
To date, legislation that would allow states to join the compact has been introduced in seven states [ [link removed] ] . The Tennessee [ [link removed] ] General Assembly and the Washington [ [link removed] ] State Legislature have both sent bills that would allow their states to join the compact to their respective governors.
To learn more about the compact, please join ADEA AGR for on Thursday at 1:00 p.m. for our quarterly ADEA Advocacy Insights call Matt Shafer from the National Center for Interstate Compacts and Dr. Cecille Feldman Dean of the Rutgers School of Dental Medicine, will discuss the compact.
Class Action Settlement Results in Increased Dental Coverage for New York Adults Enrolled in Medicaid
A settlement [ [link removed] ] to a class action will lead to increased dental coverage for nearly 5 million New York adults enrolled in Medicaid. Under the settlement, the state will provide coverage for implants, root canals and replacement dentures.
Prior to the settlement, the state covered some of the procedures under limited conditions. Plaintiffs in the suit argued that Medicaid enrollees were being denied medically necessary treatment [ [link removed] ] . Although dental coverage for adults falls under an optional category of care for state Medicaid programs, federal law requires states to provide coverage for all medically necessary care that is included under an optional category for which the state chooses to provide care.
Maryland Makes Changes to Faculty Licensure for Foreign-trained Dentists
Maryland Gov. Wes Moore (D) recently signed a bill [ [link removed] ] that made several changes to requirements for foreign-trained dentists who are seeking a faculty license to teach at the University of Maryland School of Dentistry. Under the new law, foreign-trained dentists can qualify for a faculty license if they are:
• A board certified by the American Board of General Dentistry or
• A general dentist who has successfully completed a general dental practice residency or advanced education in a general dentistry program.
Prior to the bill signing, a foreign-trained dentist could only qualify for a faculty if they completed training and received a license in a dental specialty program and they were certified in a dental specialty recognized by the Maryland State Board of Dental Examiners.
ADEA Advocacy in Action
This appears weekly in the ADEA Advocate to summarize and provide direct links to recent advocacy actions taken by ADEA. Please let us know what you think and how we might improve its usefulness.
Issues and Resources
• Applications open [ [link removed] ] for Ryan White Part F Dental Reimbursement Program
• Applications open [ [link removed] ] for HRSA Dental Public Health Research Fellowship
• ADEA report [ [link removed] ] on teledentistry
• ADEA report [ [link removed] ] on the Impact of the COVID-19 Pandemic on U.S. Dental Schools
• ADEA policy brief [ [link removed] ] regarding overprescription of antibiotics
• For a full list of ADEA memos, briefs and letters click here [ [link removed] ] .
Key Federal Issues [ [link removed] ]
ADEA U.S. Interactive Legislative and Regulatory Tracking Map [ [link removed] ]
Key State Issues [ [link removed] ]
The ADEA Advocate [ [link removed] ] is published weekly. Its purpose is to keep ADEA members abreast of federal and state issues and events of interest to the academic dentistry and the dental and research communities.
©2023
American Dental Education Association
655 K Street, NW, Suite 800
Washington, DC 20001
Tel: 202-289-7201
Website: www.adea.org [ [link removed] ]
[[link removed]]
Unsubscribe
[link removed]
Subscribe
[link removed][0]&p_colname=p_last_nm&p_varname=p_val_arr[1]&p_colname=p_alias&p_varname=p_val_arr[2]&p_colname=p_login_id&p_varname=p_val_arr[3]&p_colname=p_passwd&p_context=NEWSLETTER&p_success_url=censsaindprofile.section_update%3Fp_profile_ty%3DINDIVIDUAL_PROFILE%26p_skip_confirm_fl%3DY%26p_section_nm%3DNewsletters%26p_format%3D110%26p_msg_txt%3D%26p_cust_id%3D%26p_referrer%3D
B. Timothy Leeth, CPA
ADEA Chief Advocacy Officer
Bridgette DeHart, J.D.
ADEA Director of Federal Relations and Advocacy
Phillip Mauller, M.P.S.
ADEA Director of State Relations and Advocacy
Brian Robinson
ADEA Program Manager for Advocacy and Government Relations
Contact Us:
[email protected] [ [link removed] ]
Powered by Higher Logic [link removed]
Message Analysis
- Sender: American Dental Education Association (ADEA)
- Political Party: n/a
- Country: United States
- State/Locality: n/a
- Office: n/a
